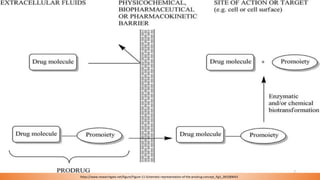
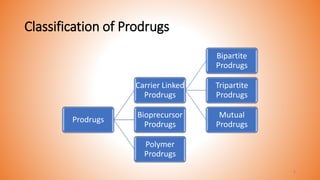
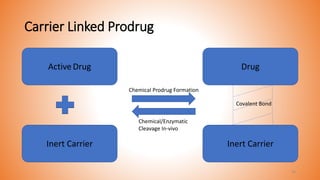
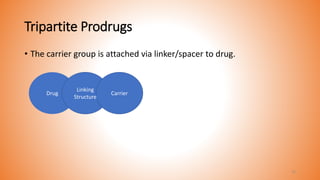
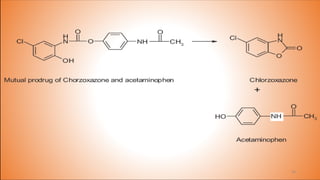
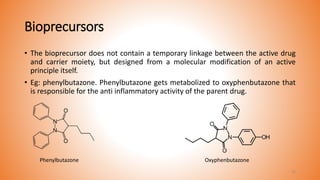
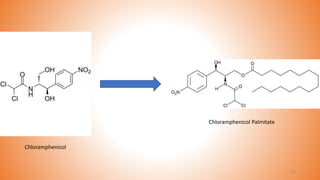
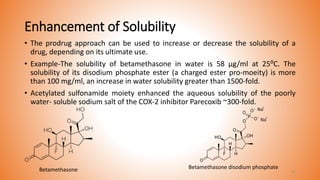
This document provides an overview of prodrug design. It defines a prodrug as an inactive derivative of a drug molecule that undergoes biotransformation to release the active drug. Prodrugs are classified based on their structure and include carrier-linked, bipartite, tripartite, mutual, and bioprecursor prodrugs. The document discusses various rationales for prodrug design such as improving solubility, absorption, patient acceptability, and site-specific drug delivery. Common functional groups used in prodrugs include esters, amides, phosphates, and carbamates. The document also covers practical considerations and approaches for overcoming limitations like pre-systemic metabolism and blood-brain barrier penetration.
























![Prodrugs With Amides
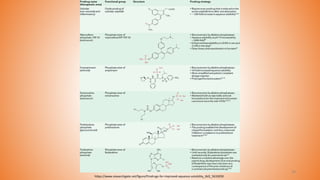
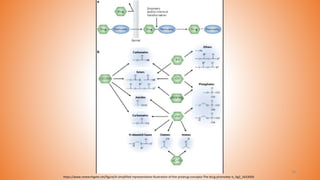
• The amide prodrugs are also used for increasing aqueous solubility of parent drug
and its bioavailability.
• Compared to esters, amide bonds are more stable to enzymatic hydrolysis.
• (a) DW2282 (26) is chemically (S)-1-[1-(4-aminobenzoyl)-2,3-dihydro-1H- indol-6-
sulphonyl]-4-phenyl-imidazolidin-2-one, which is an anticancer drug with low
water solubility (0.024 mg/mL) and higher gastrointestinal toxic effects.
• Many amino acid prodrugs were synthesized almost all of them attained higher
water solubility as compared to the parent drug.
• One of the compound have shown very good aqueous solubility (0.865 mg/mL)
and bioavailability by oral route.
38](https://image.slidesharecdn.com/prodrugdesign-201207125547/85/Prodrug-Design-38-320.jpg)
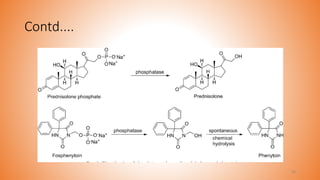
![Prodrugs With Phosphates
• The phosphate prodrugs have been proven to increase the aqueous solubility and
bioavailability of the parent drug.
• Phosphate prodrugs get converted to its parent drug by the action of intestinal
alkaline phosphatase enzyme.
• A prodrug of benzimidazole derivative α-6-chloro-2-(methylthio)-5-(napthalen-1-
yloxy)-1H- benzo[d] imidazole. The prodrug synthesized by linking disodium
phosphate and found be 50,000-folds higher water soluble than the parent drug.
39](https://image.slidesharecdn.com/prodrugdesign-201207125547/85/Prodrug-Design-39-320.jpg)