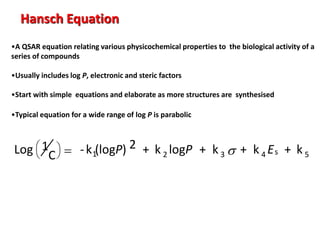
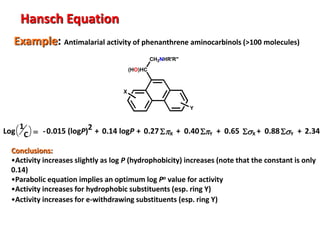
Quantitative Structure Activity Relationships (QSAR) relates the biological activity of compounds to their physicochemical properties using mathematical models. QSAR equations express how properties like hydrophobicity, electronic effects, and steric hindrance of molecules and substituents influence biological response. Hydrophobicity is often quantified by log P values, while electronic and steric effects use constants like Hammett's s, Taft's Es, and molar refractivity. Successful QSAR equations incorporate multiple physicochemical terms to predict activity for new structures within the same class.


![Mathematical Model
•Biological activity can be expressed quantitatively as the concentration of a
substance required to give a certain biological response.
•Additionally, when physicochemical properties are expressed by numbers, one
can form a mathematical relationship, i.e. QSAR, between the two.
•The mathematical model can then be used to predict the biological response
of other chemical structure.
•QSAR has a form of mathematical
•Biological activity is normally expressed as 1/C, where C = [drug] required to
achieve a defined level of biological activity
Activity = ƒ (Physicochemical properties) + Error](https://image.slidesharecdn.com/qsar6thsem-210510062343/85/QSAR-Quantitative-Structural-Activity-Relationship-3-320.jpg)
![Hydrophobicity of the Molecule
Partition Coefficient P = [Drug in octanol]
[Drug in water]
High P High hydrophobicity](https://image.slidesharecdn.com/qsar6thsem-210510062343/85/QSAR-Quantitative-Structural-Activity-Relationship-4-320.jpg)

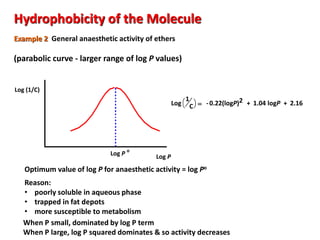
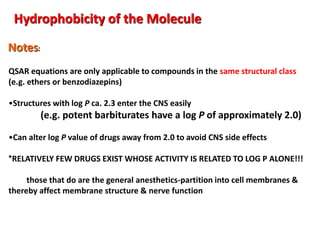

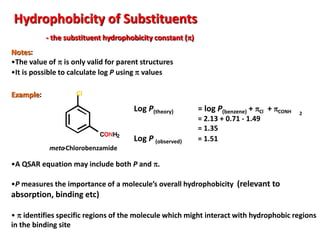
![Electronic Effects
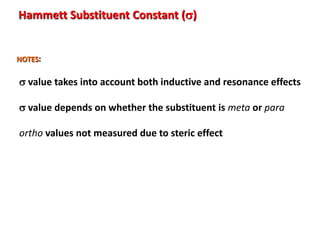
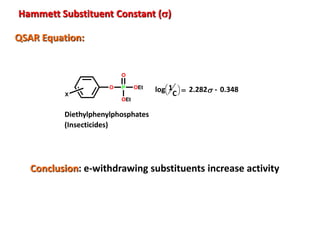
Hammett Substituent Constant (s)
Notes:
•The constant (s) is a measure of the e-withdrawing or e-donating influence of
substituents
•It can be measured experimentally and tabulated
(e.g. s for aromatic substituents is measured by comparing the
dissociation constants of substituted benzoic acids with
benzoic acid)
X=H K H = Dissociation constant= [PhCO 2-]
[PhCO 2H]
+
CO2H CO2 H
X X](https://image.slidesharecdn.com/qsar6thsem-210510062343/85/QSAR-Quantitative-Structural-Activity-Relationship-10-320.jpg)