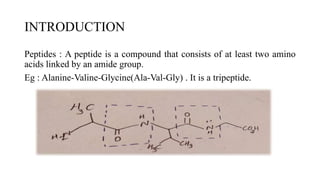
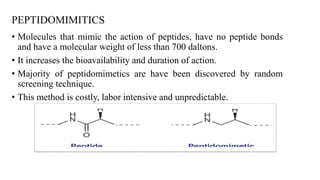
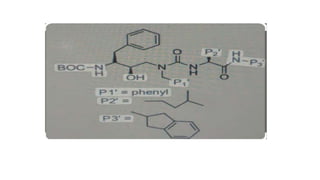
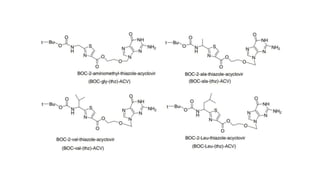
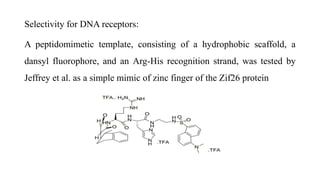
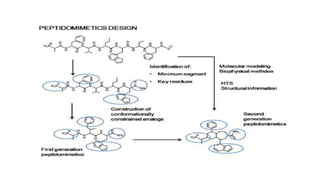
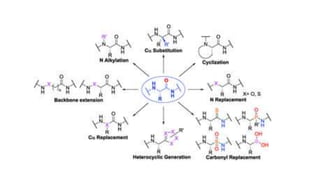
This document discusses peptidomimetics, which are molecules that mimic peptides but do not contain peptide bonds. Peptidomimetics have increased bioavailability and duration of action compared to peptides. There are four types of peptidomimetics: Type I mimic peptide backbones, Type II are small molecules that bind peptide receptors, Type III have novel scaffolds that position essential groups, and Type IV are non-peptide molecules. Peptidomimetics have various therapeutic applications including antimicrobial, anticancer, antiviral, and antioxidant activities. They can be designed by replacing parts of peptides with non-peptide moieties or by hypothesizing the bioactive peptide form. Manipulating amino acids or their side chains allows creating