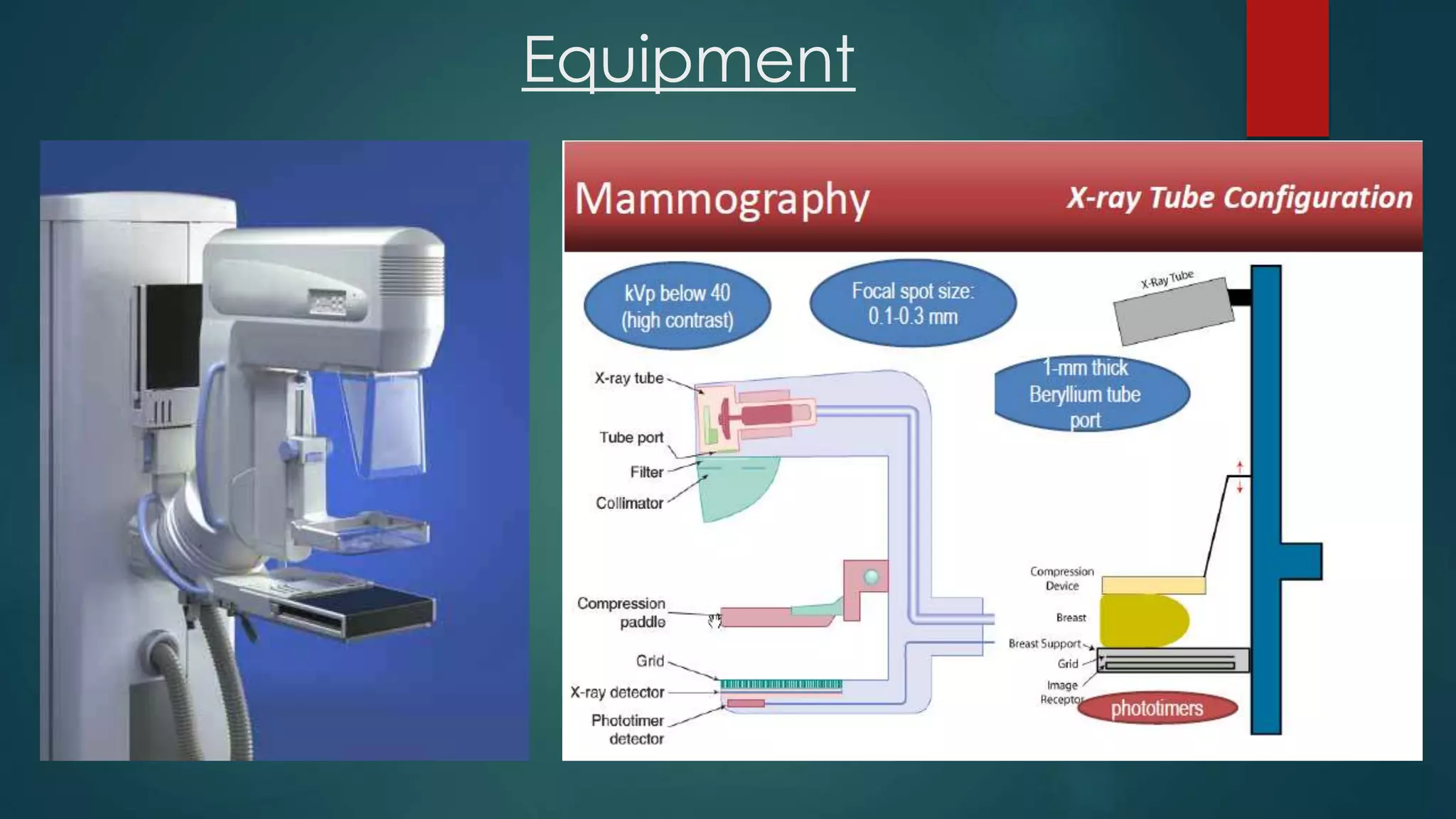
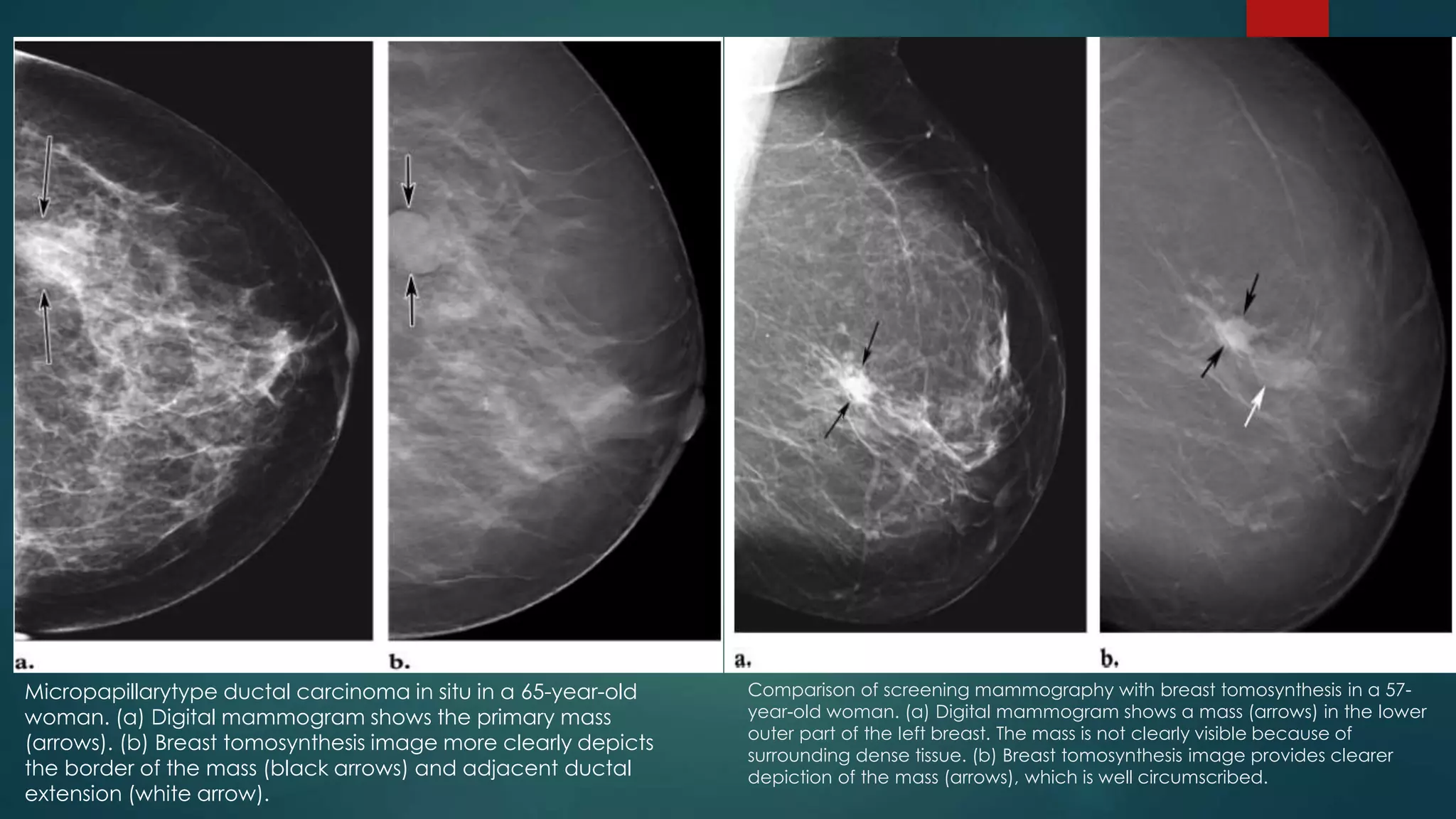
The document discusses recent advancements in mammography, highlighting the historical context, the evolution of technologies such as digital mammography, contrast-enhanced digital mammography, and breast tomosynthesis, which improve imaging quality and detection rates. It outlines the advantages and disadvantages of different methodologies, including patient comfort and sensitivity to lesions in dense breast tissue. Additionally, it emphasizes the importance of technological developments in enhancing breast cancer detection and diagnosis.


































![Scintimammography
Scintimammography, also known as nuclear medicine breast imaging, is an examination that may be
used to investigate a breast abnormality that has been discovered on mammography.
Scintimammography is also known as Breast Specific Gamma Imaging (BSGI) or Molecular Breast Imaging
(MBI).
Done in those who had abnormal mammograms, or for those who have dense breast tissue, post-
operative scar tissue or breast implants.
Patient receives an injection of a small amount of a radioactive substance called technetium 99
sestamibi, which is taken up by cancer cells, and a gamma camera is used to take pictures of the breasts.
Also called a Miraluma test (when with sestamibi)[3] and sestamibi breast imaging.
The procedure is less accurate in evaluating abnormalities smaller than one centimeter.
Patient is exposed to slightly more radiation than mammography but has higher sensitivity and PPV than
conventional mammography.](https://image.slidesharecdn.com/recentadvancesinmammography-150410103808-conversion-gate01/75/Recent-advances-in-Mammography-38-2048.jpg)






























![Refernces :
Diekmann F., Bick U: "Tomosynthesis and contrast-enhanced digital mammography:
recent advances in digital mammography"; European Radiology; v.17:3086-3092
Brem, R. et al : "Detection of Occult Foci of Breast Cancer Using Breast-Speci#c Gamma
Imaging in Women with One Mammographic or Clinically Suspicious Breast Lesion";
Acad Radiol; 17:735-743
Dean, J. : "Using Automated Breast Ultrasound to Reduce or Eliminate Interval Cancers" in
www.diagnosticimaging.com
Gennaro G. et al : "Digital breast tomosynthesis versus digital mammography: a clinical
performance study"; European Radiology; v.20:1545-1553.
Weigert et al : "Results of a Multicenter Patient Registry to Determine the Clinical Impact
of Breast-Specific Gamma Imaging, a Molecular Breast Imaging Technique"; AJR:198.
Potente, G. et al : "Practical application of contrast-enhanced magnetic resonance
mammography [CE-MRM] by an algorithm combining morphological and
enhancement patterns"; Computerized Medical Imaging and Graphics 33; 83-90.](https://image.slidesharecdn.com/recentadvancesinmammography-150410103808-conversion-gate01/75/Recent-advances-in-Mammography-69-2048.jpg)