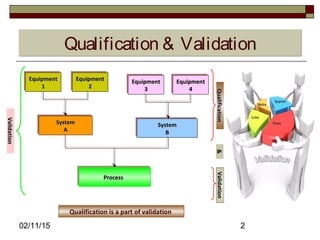
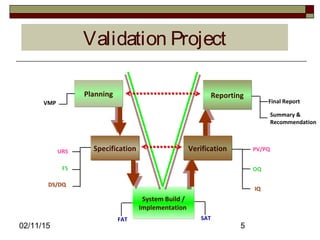
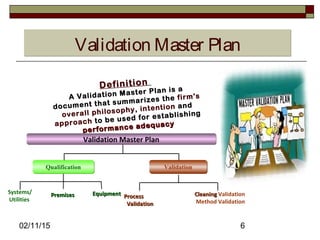
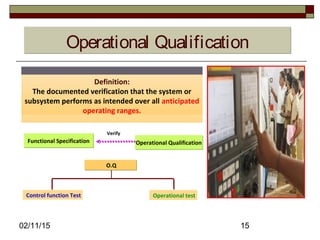
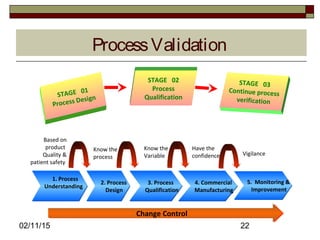
The document discusses concepts and terminology related to qualification and validation of equipment and systems. It defines qualification as proving that premises, systems and equipment are properly installed and work correctly. Validation is defined as proving that processes, procedures or methods consistently lead to expected results. The document provides definitions and descriptions of key terms in validation including validation master plan, user requirement specification, functional specification, design specification, factory acceptance testing, site acceptance testing, design qualification, installation qualification, operational qualification, process/performance qualification, protocols, reports, and types of validation.