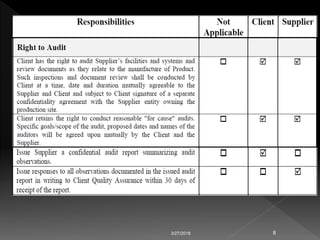
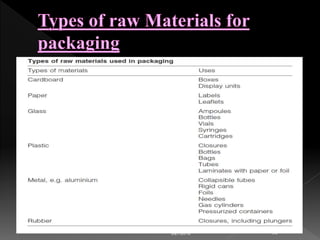
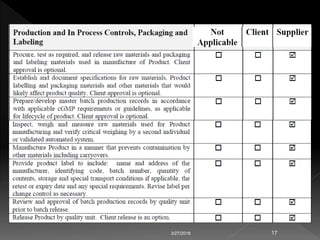
This document discusses regulations regarding the manufacture of pharmaceutical products and active ingredients, including requirements for qualifying vendors that supply materials. Strict good manufacturing practices (GMP) are required to ensure quality, safety and efficacy. Vendor qualification is important to provide assurance of drug product performance and avoid risks like contamination. The document refers to other guidance on topics like quality agreements, auditing, and assessing vendor performance on supply assurance, quality, costs, and responsiveness. Packaging component supplier audits are also discussed.