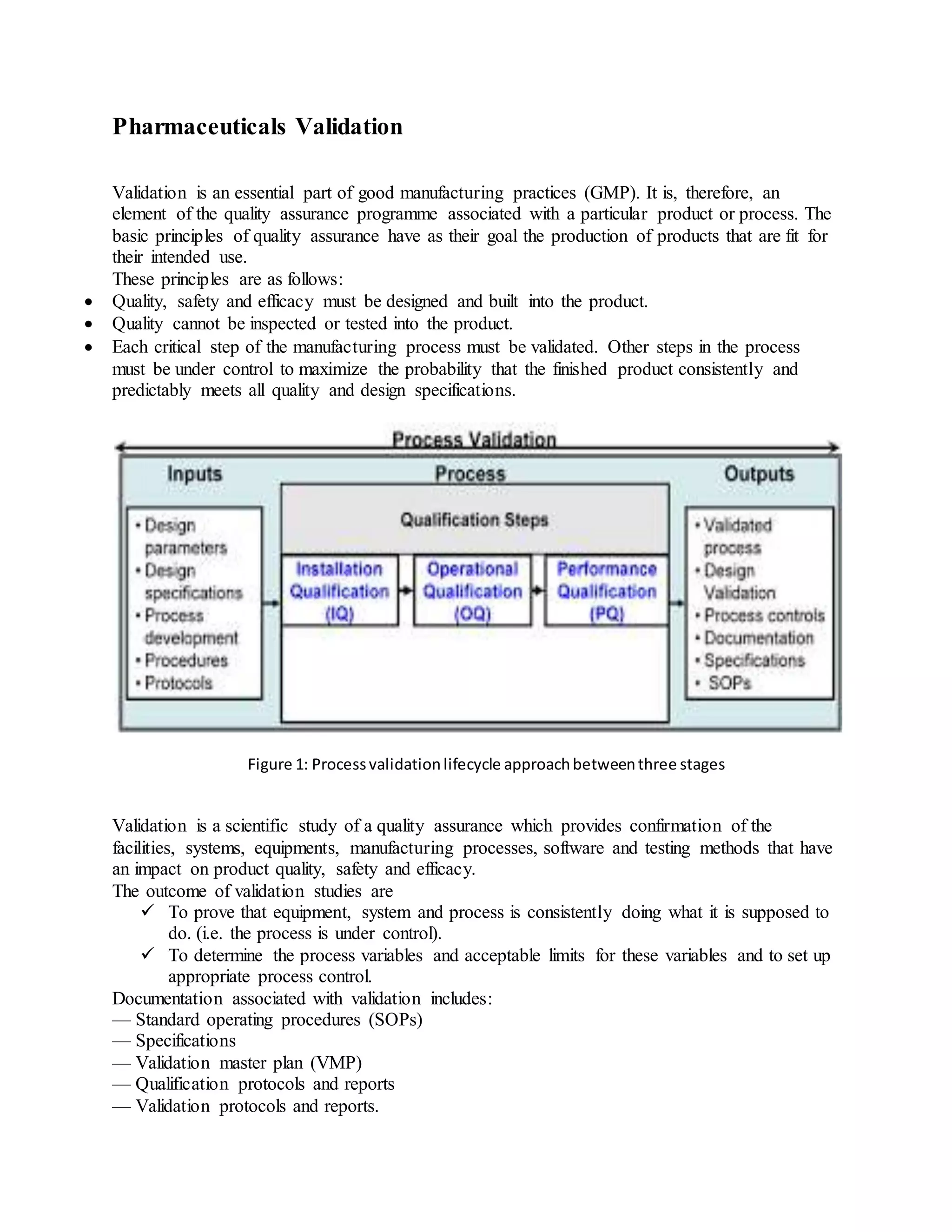
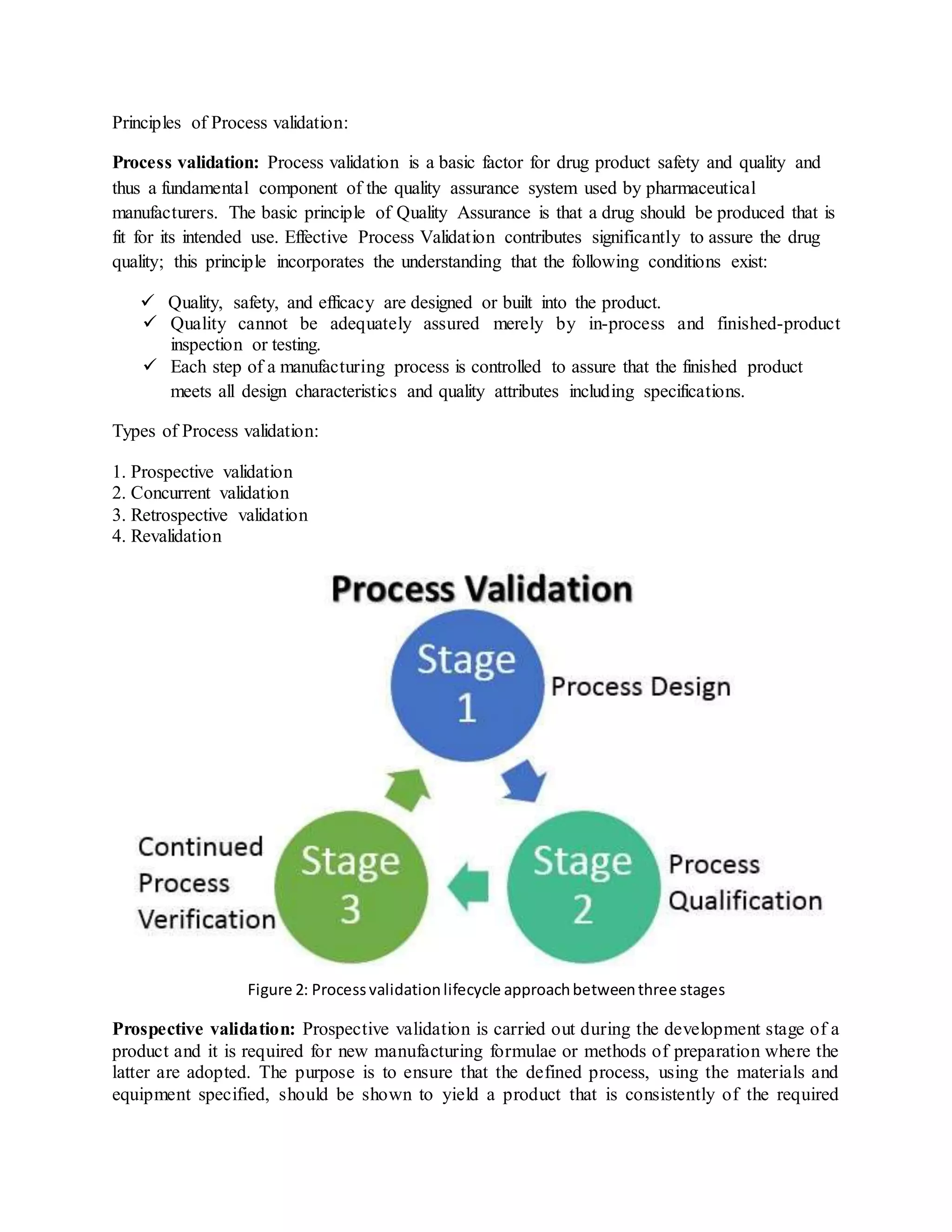
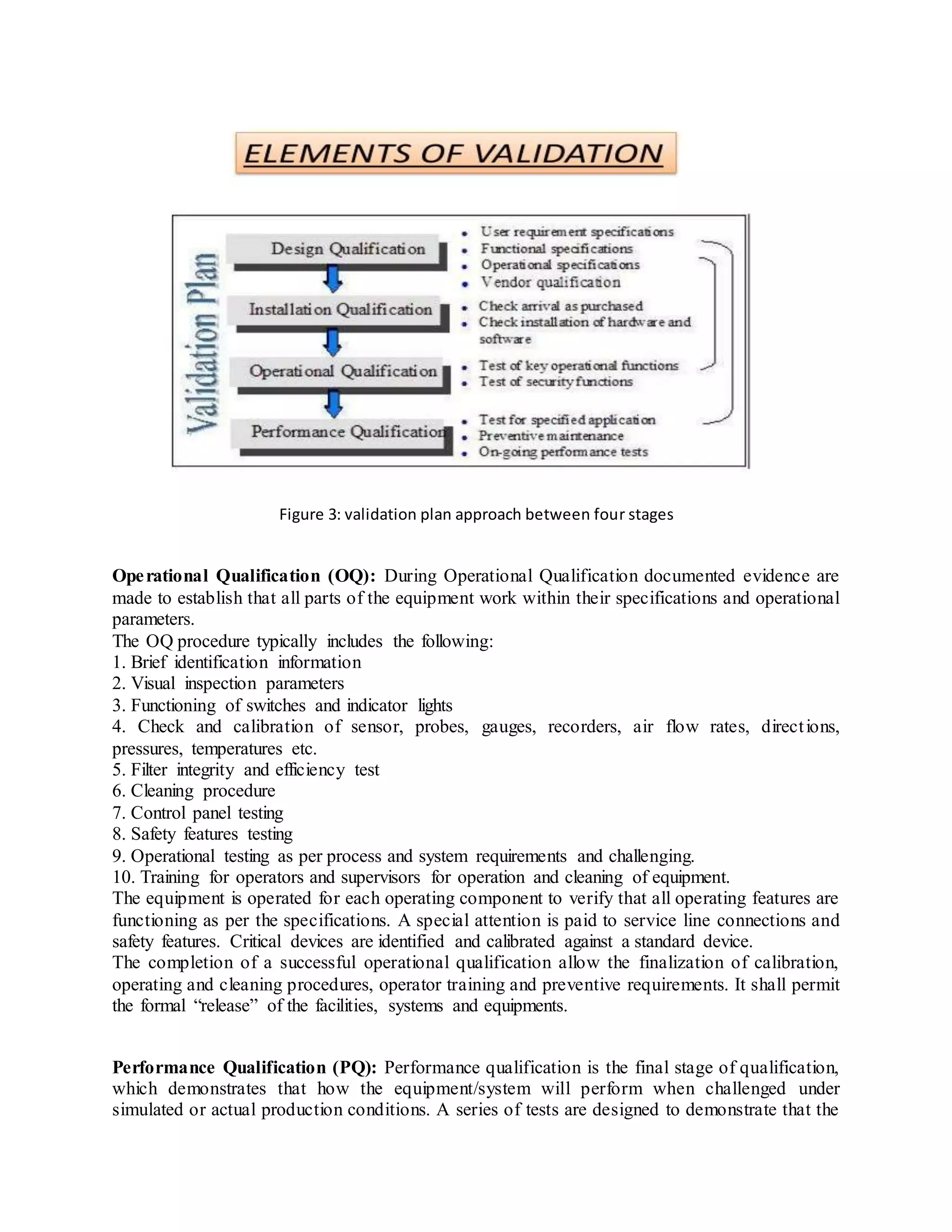
Validation is an essential part of good manufacturing practices in the pharmaceutical industry to ensure products are safe and effective. There are several types of validation including prospective validation during development, concurrent validation during production, and retrospective validation reviewing past data. Key aspects of validation include design qualification to ensure proper design, installation qualification confirming proper installation, and operational qualification to establish equipment operates as intended. The overall goal is to scientifically prove processes consistently produce quality products meeting specifications.