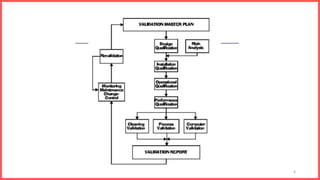
The document outlines a Validation Master Plan (VMP) which provides an overview of the validation strategy and activities for a manufacturing facility, including details on design qualification, installation qualification, operational qualification, performance qualification, personnel responsibilities, schedules, documentation requirements, and change control procedures. The VMP describes the purpose and importance of the plan for ensuring all systems, equipment, and processes are qualified and work as intended according to regulatory standards.