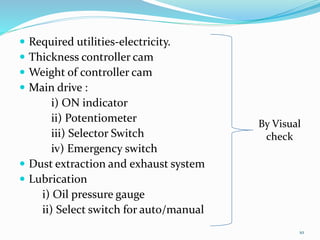
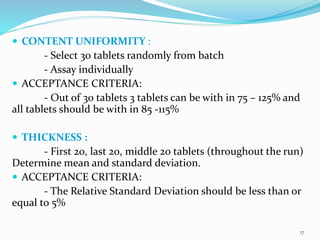
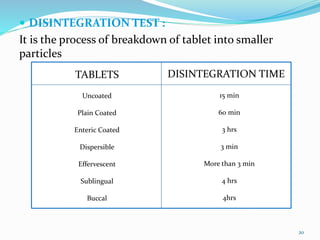
The document outlines the validation and qualification processes for tablet compression machines and capsule filling machines in the pharmaceutical industry, emphasizing the importance of installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) to ensure equipment operates correctly and produces quality products. It details the objectives and checklist items for each qualification stage, including checks for tooling, operational parameters, and physical characteristics of tablets or capsules. The document serves as a guide for ensuring compliance with regulatory requirements while assessing and maintaining production standards.