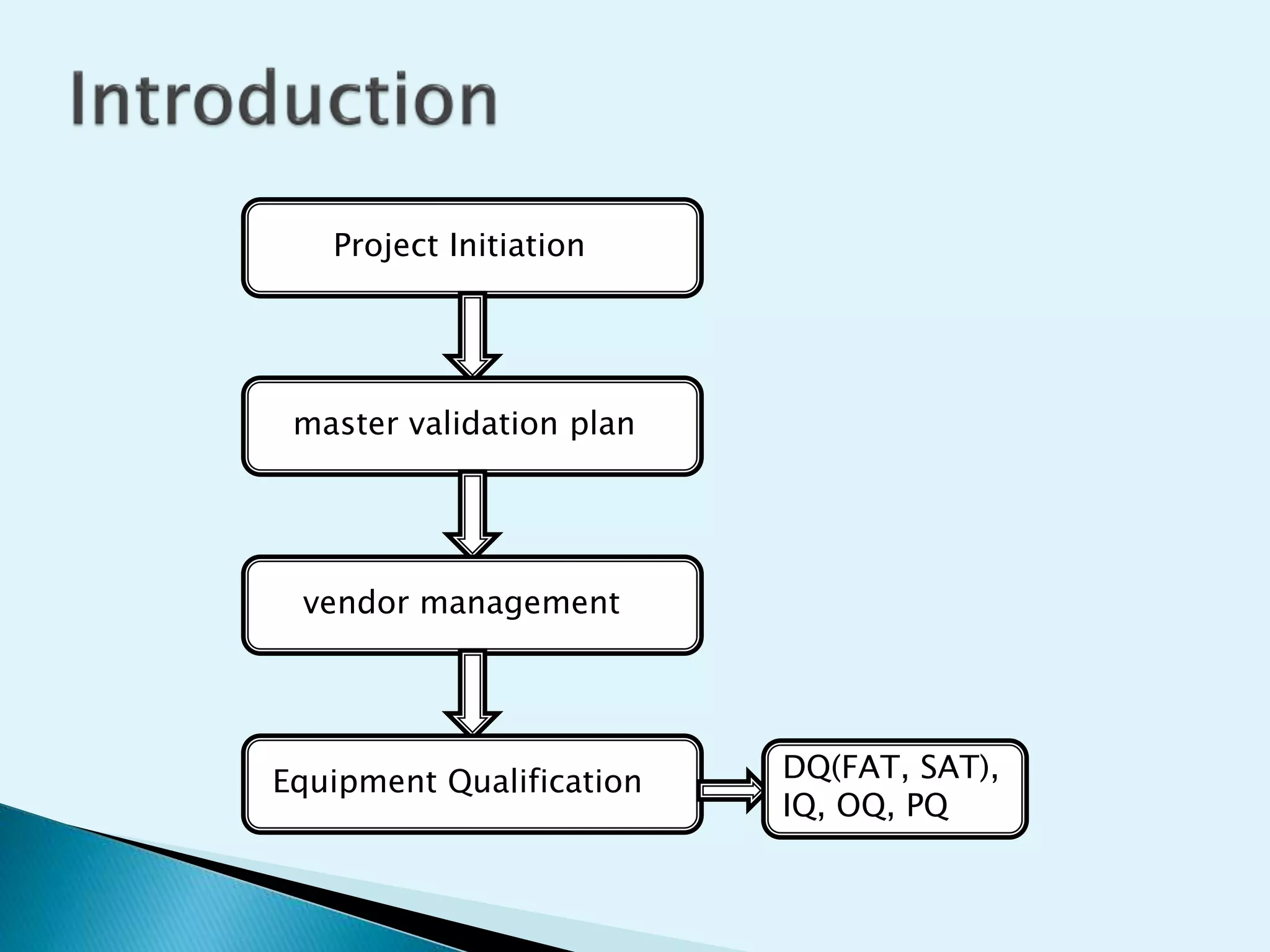
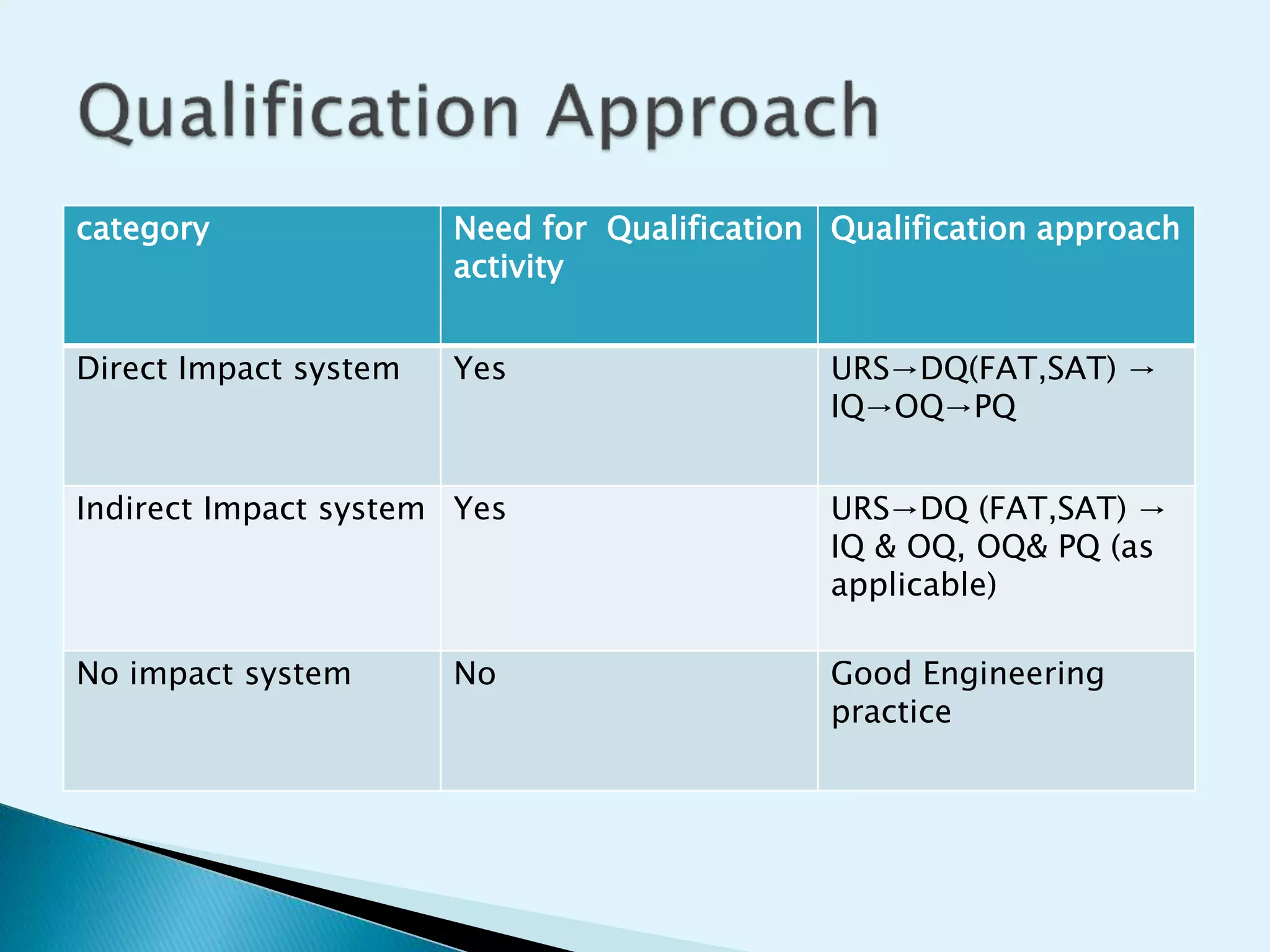
The document discusses the process of equipment qualification which includes design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). DQ establishes that the equipment design meets predefined user requirements. IQ demonstrates that equipment has been properly installed. OQ shows that equipment can consistently operate within defined parameters. PQ shows that equipment can consistently meet performance standards under real-world conditions. The document provides details on the documentation and tests required for each qualification step.