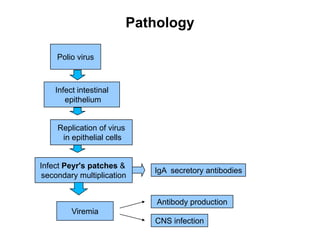
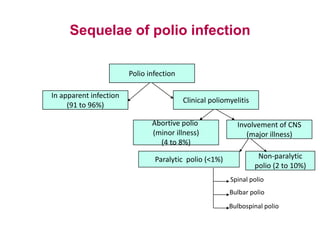
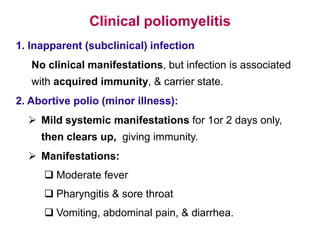
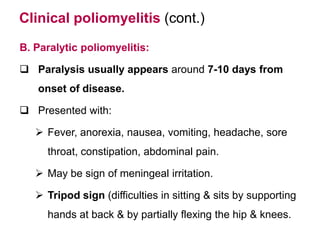
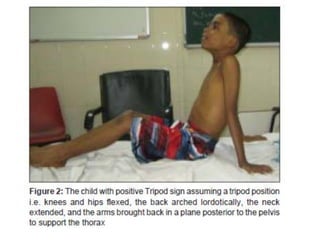
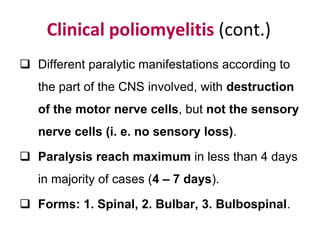
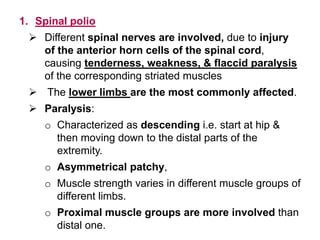
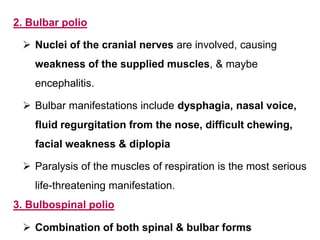
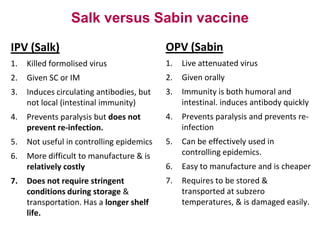
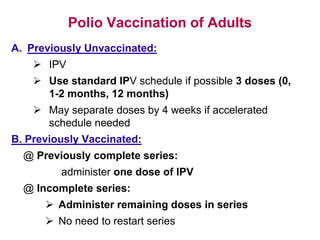
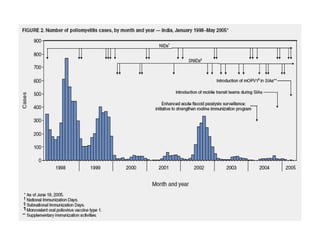
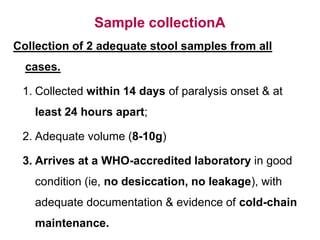
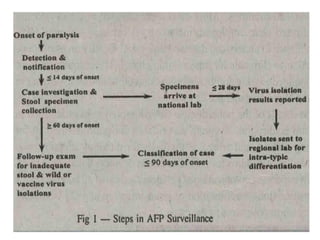
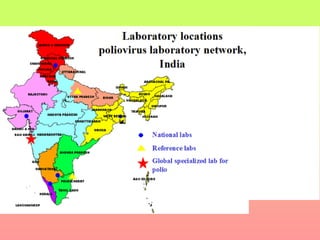
Poliomyelitis is a highly infectious disease caused by the poliovirus. While most infections are asymptomatic, it can cause paralysis in about 1% of cases. The document discusses the epidemiology, clinical manifestations, diagnosis, treatment and prevention of polio through vaccination. It provides details on the inactivated polio vaccine and oral polio vaccine, and strategies used in India's polio eradication program such as intensified pulse polio immunization campaigns and acute flaccid paralysis surveillance. The goal is to replace wild poliovirus circulation with vaccine-derived poliovirus until transmission is stopped globally.