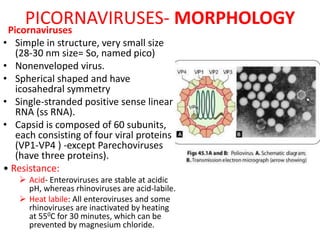
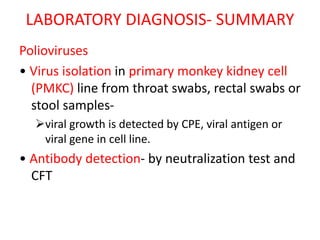
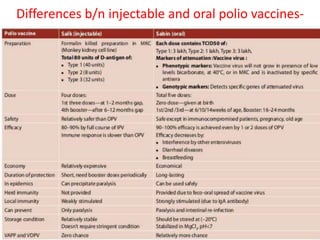
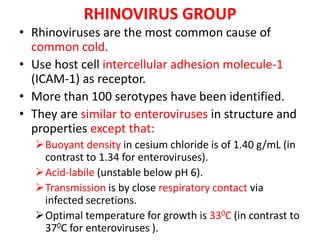
Picornaviruses are a family of small, non-enveloped viruses that includes enteroviruses and rhinoviruses. Enteroviruses such as poliovirus, coxsackievirus, and echovirus can cause diseases ranging from the common cold to meningitis, hand-foot-and-mouth disease, and even paralysis. Poliovirus is classified into three serotypes and can be diagnosed through virus isolation from throat or stool samples or antibody detection in serum. Both injectable inactivated and oral live attenuated vaccines are used to protect against poliovirus. Global vaccination efforts have nearly eradicated polio, with transmission now only occurring in a few countries.