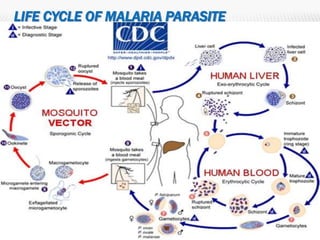
The document provides an overview of malaria epidemiology, prevention, and control efforts in India. It discusses that malaria affects millions of people annually in India, transmitted primarily by Anopheles mosquitoes. Key prevention strategies mentioned include vector control through indoor residual spraying and larviciding, and prompt diagnosis and treatment of cases. Major control programs launched over time aimed to reduce malaria incidence and mortality, through activities like active case detection, radical treatment, and insecticide spraying. National strategies have evolved from eradication to control efforts as challenges emerged.