This document provides an overview of polio, including:
1. A brief history of polio and its identification and naming.
2. Details on poliovirus itself, including that it is an enterovirus with three serotypes that are targeted by vaccines.
3. The pathogenesis of poliovirus and how it typically enters the body and can in rare cases affect the nervous system and cause paralysis.
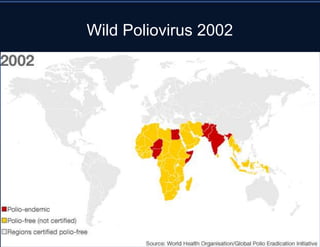
4. Epidemiological details on polio including types of infection outcomes and its historic prevalence prior to vaccination efforts.
5. Prevention strategies including the global polio eradication initiative and details on the inactivated and oral polio vaccines used in vaccination programs.