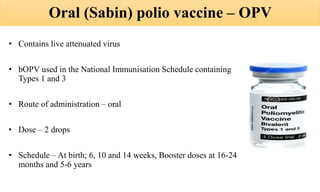
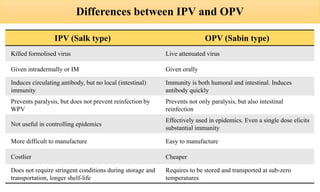
This document discusses poliomyelitis (polio), including its epidemiology, transmission, clinical presentation, diagnosis, treatment and prevention through vaccination. It notes that polio is caused by an RNA virus that primarily infects the gastrointestinal tract but can infect the central nervous system in rare cases, potentially causing paralysis or death. It describes polio vaccines including both inactivated polio vaccine (IPV) and oral polio vaccine (OPV), and mass immunization strategies like pulse polio immunization. Surveillance of acute flaccid paralysis is also summarized as a key part of global polio eradication efforts.