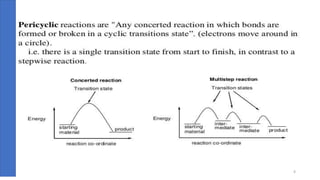
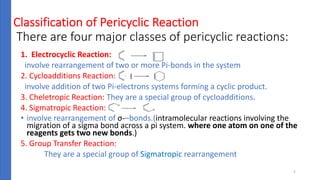
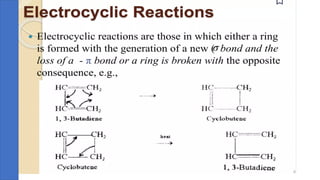
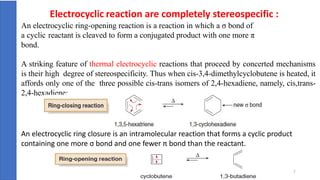
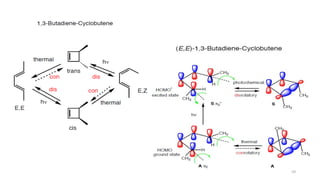
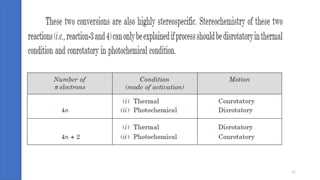
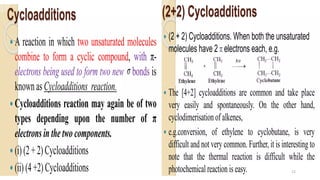
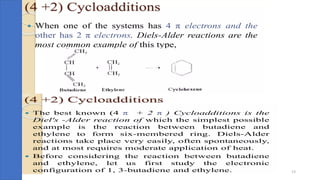
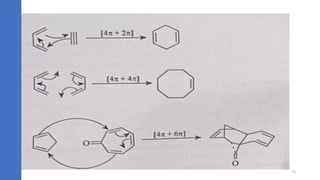
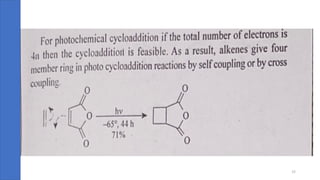
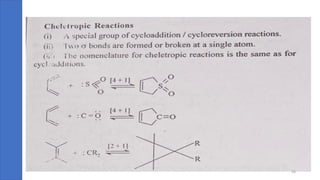
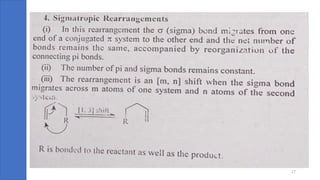
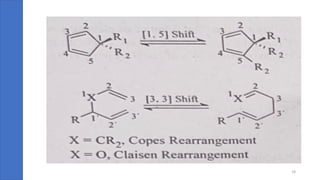
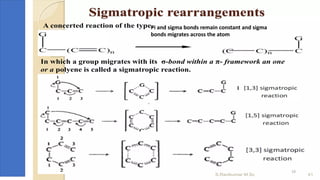
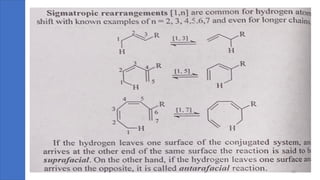
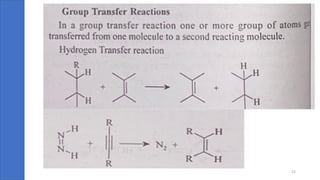
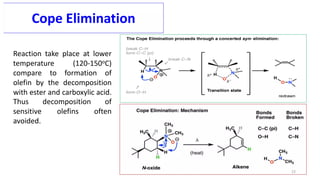
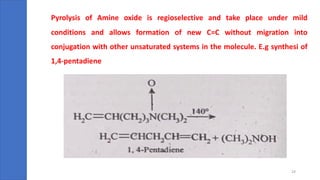
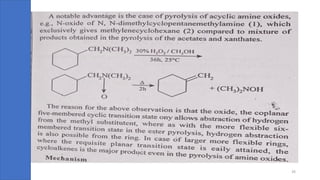
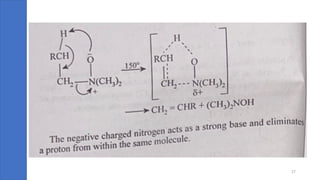
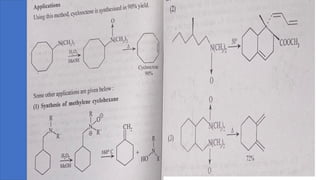
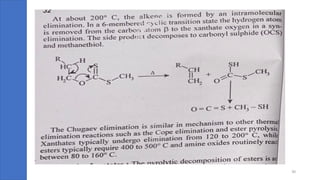
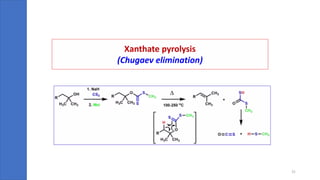
Pericyclic reactions are concerted organic reactions characterized by a cyclic flow of electrons with high stereo selectivity and no intermediates. They can be classified into four major types: electrocyclic reactions, cycloadditions, cheletropic reactions, and sigmatropic reactions, each involving unique rearrangements of bonds. Key features include reversibility, simultaneous bond breaking and formation, while their mechanisms are influenced by heat and light.