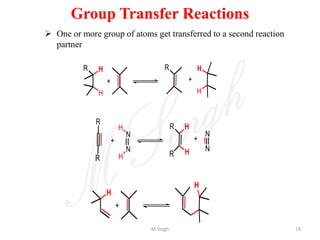
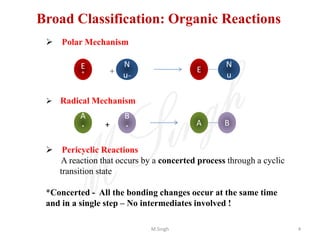
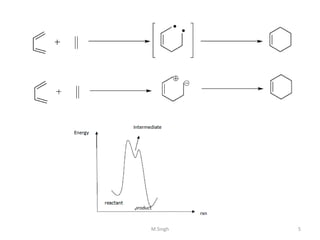
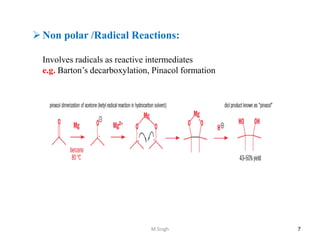
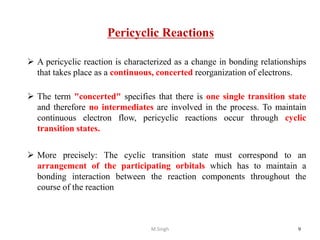
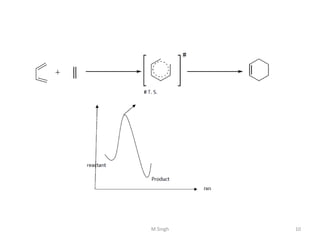
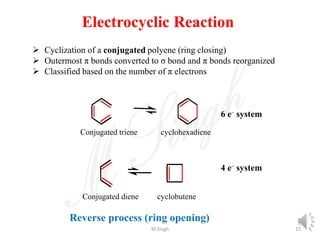
1) Pericyclic reactions are single-step, concerted reactions that proceed through a cyclic transition state without intermediates.
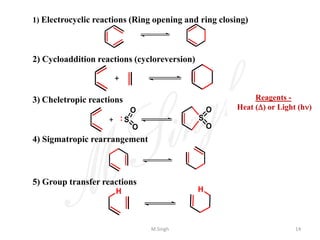
2) They are classified as electrocyclic reactions, cycloaddition reactions, cheletropic reactions, sigmatropic rearrangements, or group transfer reactions depending on the type of bonding changes that occur.
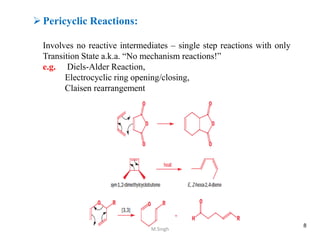
3) Examples of pericyclic reactions include the Diels-Alder reaction, electrocyclic ring openings and closings, and the Claisen rearrangement.







![Cycloaddition Reaction
[4π + 2π][2π + 2π]
[4π + 4π]
[4π + 6π]
➢ Condensation of two (or more) small π systems to form a ring
➢ σ bonds formed at the expense of π bonds
➢ Classified by the number of π electrons interacting
Cycloreversion is the reverse of cyclo addition
16M.Singh](https://image.slidesharecdn.com/pericyclicreactionpart-1-converted-200614100156/85/Pericyclic-reaction-part-1-converted-16-320.jpg)
![Cheletropic Reaction
➢ A special type of cycloaddition/cyclo-reversion reactions.
➢ Two bonds are formed or broken at a single atom.
➢ Nomenclature same as for cycloadditions.
[4+1]
[2+1]
17M.Singh](https://image.slidesharecdn.com/pericyclicreactionpart-1-converted-200614100156/85/Pericyclic-reaction-part-1-converted-17-320.jpg)
![➢ Involves the breaking and migration of a sigma bond over p
electron systems
➢ Formation of a new sigma bond occurs with reorganization of
the p systems
X = C; Cope Rearrangement
X = O; Claisen Rearrangement
Classified as [i,j] shift, because
the rearrangement occurred
when the σ-bond migrates across
i atoms of one system and j of
another
Sigmatropic Rearrangement
18M.Singh](https://image.slidesharecdn.com/pericyclicreactionpart-1-converted-200614100156/85/Pericyclic-reaction-part-1-converted-18-320.jpg)