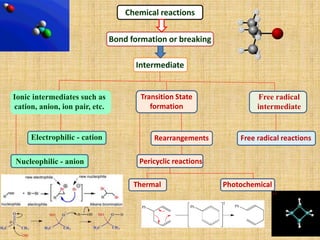
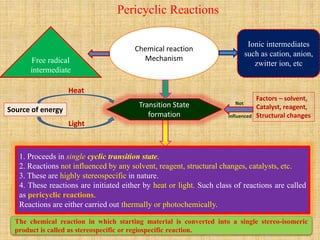
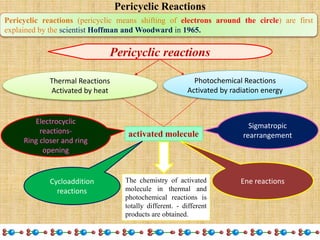
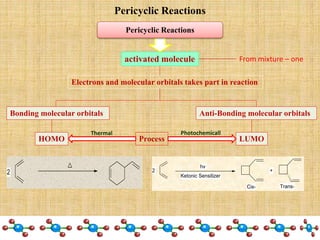
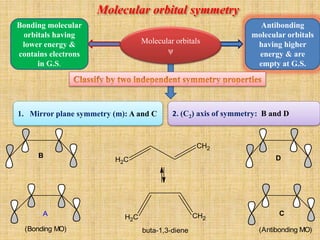
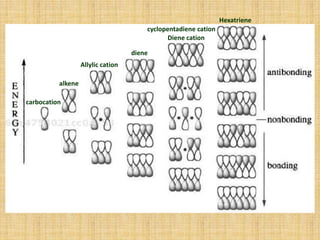
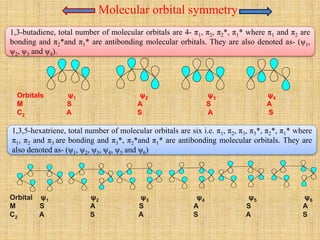
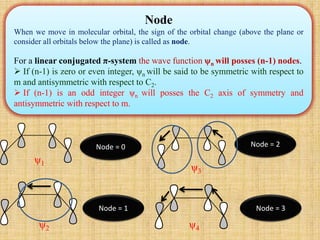
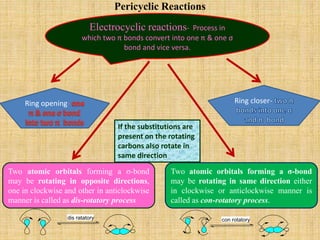
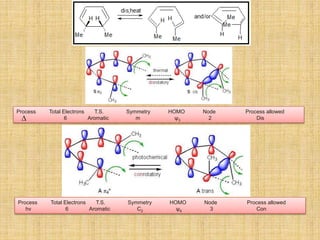
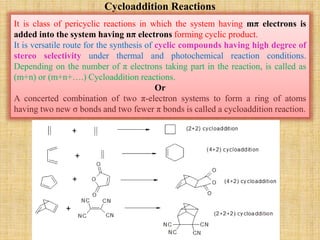
1. Cycloaddition reactions involve the addition of two pi systems to form a cyclic product with two new sigma bonds and two fewer pi bonds. They can occur suprafacially or antrafacially.
2. The Diels-Alder reaction is a common [4+2] cycloaddition between a diene and an alkene. The sign of the frontier orbitals must match for the reaction to be thermally or photochemically allowed.
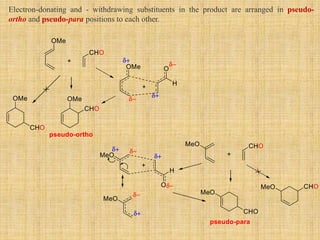
3. The diene typically has electron-donating groups and the dienophile electron-withdrawing groups for efficient Diels-Alder reactions. The stereochemistry of substituents is maintained in the product.



![Frontier Molecular Orbital [FMO] Method
Molecular orbital model for analyzing pericyclic reactions has been proposed by Kenichi
Fukui of Japan.
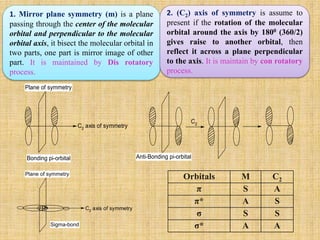
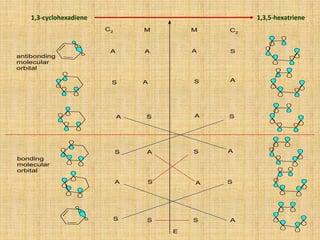
The correlation diagram is useful for the detail analysis of an Electrocyclic reactions.
A
B
C
D
X
Y
W
Z
CH2CH2
A
B
C
D
X
Y
W
Z
Bonding MO
Antibonding MO
Mirror (m) of symmetry
C2 axis of symmetry
A
S
A
S
A
S
A
S
A
B
C
D
X
Y
W
Z
A
S
A
S A
S
A
S
1,3-butadiene cyclobutene](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-14-320.jpg)
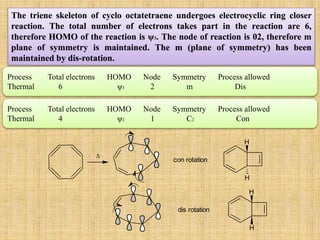
![Frontier Molecular Orbital [FMO] Method
The correlation diagram is useful for the detail analysis of an Electrocyclic reactions.
A similar conclusion is obtained by considering the symmetry of highest occupied
molecular orbital [HOMO] of open chain partner in Electrocyclic reactions.
If the HOMO having C2-axis of symmetry (node is odd), then reaction will follow con-
rotatory path.
If HOMO posses a mirror plane symmetry (node is zero or even number), a reaction will
follows dis-rotatory path.
Thermal
Reactions
Transition State Configurational Preference
4n + 2 (aromatic) Disrotatory
4n (antiaromatic) Conrotatory
Photochemical
Reactions
Transition State Configurational Preference
4n + 2 (aromatic) Conrotatory
4n (antiaromatic) Disrotatory](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-16-320.jpg)
![Frontier Molecular Orbital [FMO] Method
A similar conclusion is obtained by considering the symmetry of highest occupied molecular
orbital [HOMO] of open chain partner in Electrocyclic reactions.
A
B
C
D
X
Y
W
Z
CH2CH2
A
B
C
D
X
Y
W
Z
Bonding MO
Antibonding MO
Mirror (m) of symmetry
C2 axis of symmetry
A
S
A
S
A
S
A
S
A
B
C
D
X
Y
W
Z
A
S
A
S A
S
A
S
Thermal
HOMO
Con-rotatory](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-17-320.jpg)
![Frontier Molecular Orbital [FMO] Method
A similar conclusion is obtained by considering the symmetry of highest occupied molecular
orbital [HOMO] of open chain partner in Electrocyclic reactions.
A
B
C
D
X
Y
W
Z
CH2CH2
A
B
C
D
X
Y
W
Z
Bonding MO
Antibonding MO
Mirror (m) of symmetry
C2 axis of symmetry
A
S
A
S
A
S
A
S
A
B
C
D
X
Y
W
Z
A
S
A
S A
S
A
S
Photochemical
HOMO
Dis-rotatory](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-18-320.jpg)
![Cycloaddition Reactions
In cycloaddition reactions, addition of two systems having double bonds take-
place either in same or opposite side of the system. Such mode of addition is very
important to decide the stereo chemistry of product. These different numbers of
modes are named as suprafacial (on the same side) and antrafacial (on the
opposite side).
This specification is usually carried out by keeping a suitable subscript (s or a) after
the number referring to the pi-components. E.g. The Diels-Alder reaction is also
called as (4s+2s) cycloaddition.
[suprafcial] [antrafacial]
R
R
R
R
H
H
+
heat
(4s+2s)](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-30-320.jpg)
![Frontier Molecular Orbital [FMO] Method
[Cycloaddition Reactions]
A cycloaddition reaction is allowed or not which can be found to depends on the symmetry
properties of the highest occupied molecular orbital (HOMO) of one reactant and the
lowest unoccupied molecular orbital (LUMO) of other molecule.
The favorable interaction can be predicted from the sign of HOMO and LUMO.
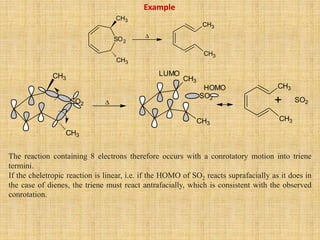
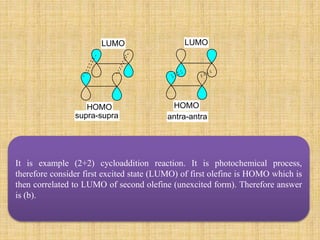
e.g. During Cycloaddition of ethylene to cyclobutane (2s+2s) addition is thermally forbidden
because lopes of HOMO of one ethylene molecule and that of other LUMO of another
ethylene molecule are not having corresponding similar signs, it (2a+2s) thermally allowed.
But when one ethylene molecule is irradiated, on electron get excited to excited state
(antibonding molecular orbital) which is now become HOMO which is then correlated to
LUMO of other unexcited ethylene molecule. Therefore, it is photochemically (2s+2s)
allowed.
HOMO
LUMO
HOMO of excited state.
LUMO of unexcited state.
(2s + 2s)
(Ground state)
[ , forbidden]
(Excited state)
[ hv, allowed]](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-31-320.jpg)
![Frontier Molecular Orbital [FMO] Method
[Cycloaddition Reactions]
In Diels-Alder reaction as sign of the 1,4-lopes of butadiene HOMO have been found to be
matching those in the LUMO of ethylene. The (4s+2s) addition is thermally allowed and
photochemically forbidden whereas (4a+2s) or (4s+2a) is photochemically allowed and
thermally forbidden.
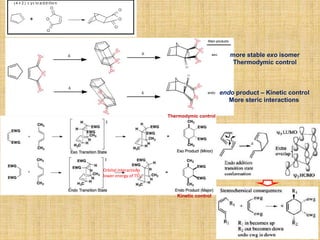
The Diels-Alder reaction of cyclpentadiene to forming dicyclopentadiene. Invariably, the
endo-dimer is formed rather that exo-dimer because of favorable secondary force due to
interaction of frontier orbitals of diene and dienophile compounds which leads to lower the
energy of the transition.
(4s + 2s, , allowed) (4s + 2s, , allowed)
(4s + 2a or 4a + 2s, hv , allowed)
HOMO
LUMO
HOMO
+
LUMO
(endo form)
(exo form)](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-32-320.jpg)
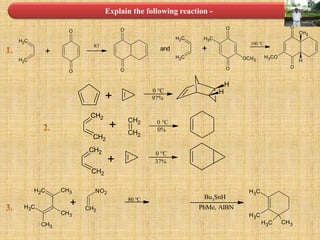
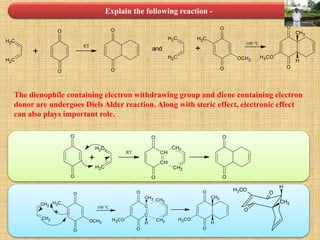
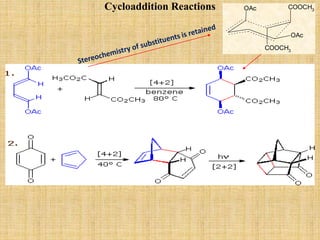
![Cycloaddition Reactions
The most common cycloaddition reaction is the [4π+2π] cyclization known as the Diels-
Alder reaction in which cyclic product is formed from alkene and a diene.
The stereochemistry of the substituent attached to double bonded carbon atom is
maintained.
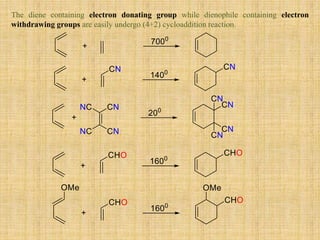
The diene containing electron donating group while dienophile containing electron
withdrawing groups are easily undergo (4+2) cycloaddition reaction.
O
O
O
CN
CNNC
NC
O
O
O
CN
CNNC
NC
+ +
(4+2) cycloaddition
(2+2+2) cycloaddition
+ one step mechanism
+ or+ - .
two step mechanism zwitterions diradicals](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-33-320.jpg)

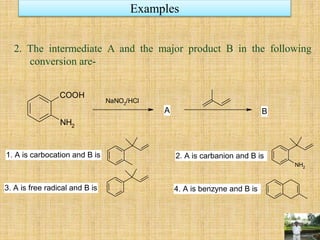
![It is combination of two reactions- Benzyne formation and Diel’s Alder reaction. The
anthranilic acid undergoes diazotizing followed by decarboxylation forming benzyne. It
shows [4+2] cycloaddition reaction [Diel’s Alder reaction] forming cyclic product (4).
NH2
COOH
NaNO2 ClH HNO2
NaCl
HNO2
N2
Cl
COOH
O
O
H
N2
Cl
+ +
+
A
+
[4+2] cycloaddition rea.
Diel's Alder reaction
B](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-42-320.jpg)
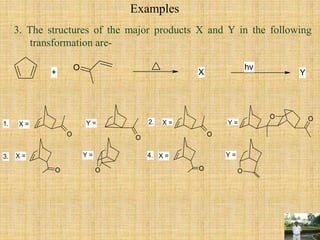
![During Diel’s Alder reaction between cyclic diene and substituted dienophile
forming endo product rather than exo because of favorable secondary force due to
interaction of frontier orbitals of diene and dienophile compounds which leads to lower
the energy of the transition (3).
The X further undergoes photochemical [2+2] cycloaddition reaction of alkene and
carbonyl group (Paterno-Buchi reaction) forming oxetane [four membered cyclic ether].](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-44-320.jpg)
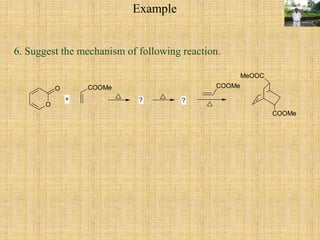
![O
O COOMe
O
O
COOMe
+Step I
HOMO
LUMO
Total electrons - 6, node- 2,
Aromatic T.S., Supra-supra
process allowed
O
O
COOMe COOMe
COOMe
CO2
or It is electrocyclic ring opening
reaction.Step II
COOMe
MeOOC
COOMe
COOMe
COOMe
+orStep III.
It is [4+2] cycloaddition reaction
called Diel's Alder reaction. The
substituent on dienophile is endo
when using cyclic diene.](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-46-320.jpg)
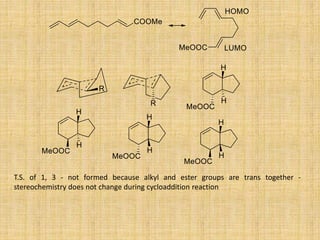
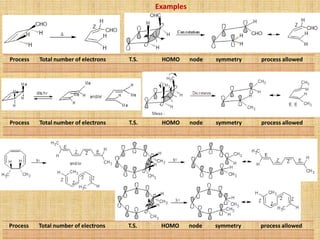
![Sigmatropic Rearrangement
Many thermal and photochemical rearrangements are known which involve the
shifting of a σ-bond flanked by one or more π-electron systems to a new position
[i,j] with in the molecule.
It is an uncatalyzed intramoleculer process.
This rearrangement involve shifting of the σ-bond, hence called as sigmatropic
rearrangement of the order [i,j]. The i and j are two number set in the square
bracket and the numbering of system is done by starting with atoms from which the
migration of the σ-bond started.
In some rearrangements, the migrating σ-bonds lie between two conjugated bond
systems. e.g. Cope & Claisen rearrangements.
R1
R1
R1
R1
e.g. 1.
2.
[1,3] shift
[1,5] shift
X
X
1
2 3
1'
2' 3'
(3,3) shift
(X - C<, O)](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-49-320.jpg)
![Sigmatropic Rearrangement
Suprafacial and Antrafacial processes
In sigmatropic rearrangement, the σ-bond migrates across the π-bonds through the
two different stereo-chemical sources.
Migrated σ-bond gets moved across the same face of the conjugated system,
suprafacial process
migrating σ-bond get reformed on the opposite π-electron face of the
conjugated system, antrafacial process i.e. migrating group migrate at opposite
face of the conjugated system.
e.g. [1,5] sigmatropic shift shows both these stereo-chemical consequences as-
BA A B
H
C D
H
C D
BA
A B
HC D
H
C D
suprafacial
antrafacial
As lengthing of the migrating system increases, antrafacial shift also increases. The
antrafacial [1,3] shift is thermally impossible.](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-50-320.jpg)
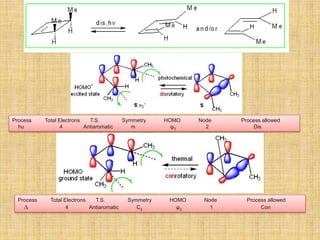
![FMO Methods (Sigmatropic Rearrangement)
The analysis of the sigmatropic rearrangement shows that the migrating bond gets
cleaved homolytically resulting pair of radicals. The migrating group get migrates
over the HOMO’s of the conjugated system.
e.g. Analysis of suprafacial [1,5] sigmatropic shift of hydrogen in which the
homolytic cleavage gives rise to the production of hydrogen atom and pentadienly
radical.
The ground state electronic configuration of pentadienly radical is ψ1
2ψ2
2ψ3
1 and
HOMO is ψ3
1 of this radical possess same sign on the terminal lopes (plane
symmetry). Therefore thermally [1,5] migration is suprafacially allowed.
H
H H
H
3](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-51-320.jpg)
![FMO Methods (Sigmatropic Rearrangement)
The first excited state electronic configuration of pentadienly radical is ψ1
2ψ2
2ψ4
1
and HOMO is ψ4
1 of this radical possess opposite sign on the terminal lopes (C2
axis of symmetry). Therefore photochemically [1,5] migration is antrafacially
allowed.
ψ1
2ψ2
2ψ3
1 ψ4
0ψ5
0 → ψ1
2ψ2
2ψ3
0 ψ4
1ψ5
0
Ground state Excited state
A similar analysis shows that, for the system [i,j] possess i+j=4n+2 electrons, the
thermal reaction should be suprafacial and photochemical process should be
antrafacial but for those cases i+j=4n, the thermal reaction should be antrafacial
and photochemical process should be suprafacial. These rules are summarized as
follow-
i+j Δ-allowed hυ-allowed
4n antrafacial suprafacial
4n+2 suprafacial antrafacial
H H
H
4](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-52-320.jpg)
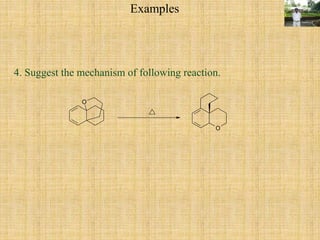
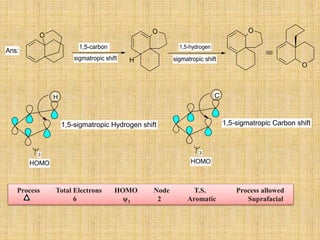
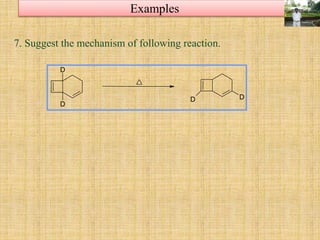
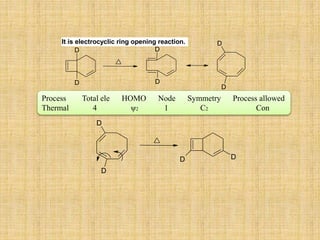
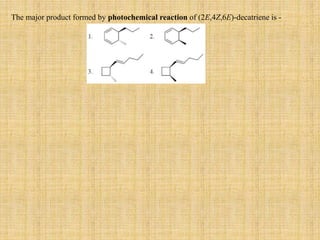
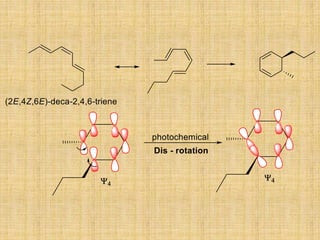
![A concerted [1,3]-sigmatropic rearrangement took place in the reaction shown below. The
structure of the resulting product is](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-55-320.jpg)

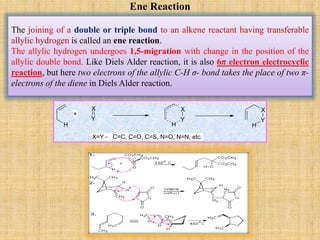
![Cope Rearrangement
The 1,5-dienes isomerizes {[3,3] rearrangement} on heating up to 3000C. Reaction
is normally reversible and gives mixture of starting material and product. The
position of equilibrium is depends on the stability of the starting material and the
products. The temperature needed for the reaction is depends on the substituents
and relative strain of the double bonds of 1,5-system. If the substituent R is
attached to double bonded carbon then energy of the transition state is lowered and
reaction occurs at 165 – 1850C.
1
2
3
1
2
3
'
'
'
[3,3] rearrangement
OH OH
O
1
2
3
1
2
3'
'
'
[3,3] rearrangement
O O
O
H
1
2
3
1
2
3'
'
'
[3,3] rearrangement +
OH O
OH
O
H
H
1
2
3
1
2
3
'
'
'
[3,3] rearrangement4.
5. [3,3] rearrangement
6. [3,3] rearrangement
100%
90%
98%
150 C, 1hr
320 C
KH, THF, reflux 18 hrs.
0
0](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-58-320.jpg)
![Cope Rearrangement
A common example of Cope rearrangement involving [3,3] sigmatropic
rearrangement in 1,5-diene (meso-3,4-dimethyl-1,5-hexadiene) on heating
(pyrolysis) giving exclusively cis,trans-isomer of 2,6-octadiene. This reaction
proceeds through six membered six electron transition state.
CH3
CH3
CH3
CH3
CH3
CH3
CH3
CH3
meso-3,4-
dimethyl-1,5-
hexadiene
Z,E-2,6-octadiene
E
Z
Bu-n
Bu-n
Bu-n
15 C
0
CH3
CH3
CH3
OH
CH3
O
CH3
CH3
CH3 CH3
CH3
OHC
KH, THF, RT
18-crown-6
CH3
CH3
CH3
OH
CH3
O
CH3
CH3
CH3
CH3
CH3
OHC
KH, THF, RT
18-crown-6](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-59-320.jpg)
![Step I is [2+2] cycloaddition reaction forming cyclobutane. It is [2s + 2s] process.
Step II is [3+3] sigmatropic rearrangement reaction called Cope rearrangement proceeds through
six membered cyclic (chair form) transition state.
H
H
hv
A HOMO HOMO
LUMO LUMO
supra-supra antra-antra
node 2, antiaromatic transition state, suprafacial allowed
H
H
H
H
H
H
A
Cyclic chair form six membered
transition state](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-61-320.jpg)
![Claisen Rearrangement
The Claisen rearrangement provides an excellent stereoselective route for the
synthesis of γ,δ-unsaturated carbonyl compounds from allylic alcohols through
allyl vinyl ethers. This reaction involves [3,3]-sigmatropic rearrangement and
takes place through a cyclic six membered transition state. The importance of
this reaction is the formation of carbon-carbon bond due to expense of carbon-
oxygen bond. It is highly stereoselective leading predominantly E-configuration of
the new double bond. The cyclic transition state preferred chair conformation with
the substituent R1 in the less hindered pseudoequatorial position.
O
R1
R2
R1
O
R2
O
R2
R1
(R2 = H, alkyl, OR, OSiR3, NR2)
Heat
The aromatic Claisen rearrangement involves [3,3] sigmatropic rearrangement of allyl
aryl ethers with migration of the allyl group (with allylic transposition) to the ortho position
of the aromatic ring. If ortho-position of the ring is substituted then double rearrangement is
occur and gives para-substituted product instead of ortho-substitution.
Br
CH3
O
OMe
Br
CH3
O
OMe
Br
CH3
OH
OMe0
190 C
decalin](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-62-320.jpg)
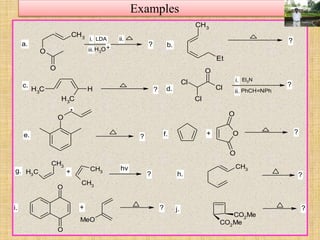
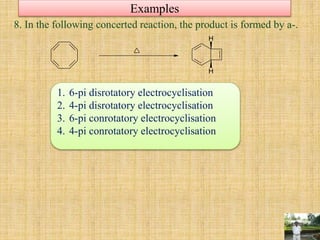
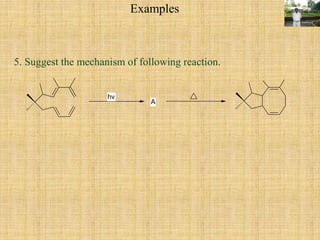
![Examples
9. The product formed and the process involved in the following
reaction is - OK
OK
O
O
O
1.
2.
3.
4.
[3,3] sigmatropic rearrangement
[1,3] sigmatropic rearrangement
[5,5] sigmatropic rearrangement
[1,5] sigmatropic rearrangement](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-63-320.jpg)

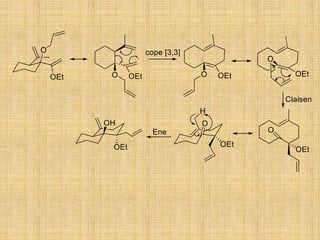
![CH2
CH2
OK
CH2
CH2
OK
CH2
CH2
OK
OK
O
O
CH2
CH2
OK
CH2
CH2
O
CH2
CH2
O
1
2
3
4
5
1
2
3
4
5
[3,3] sigmatropic rearrangement [5,5] sigmatropic rearrangement
The selection rule for sigmatropic rearrangement is –
The total number of electron involved for the sigma-tropic rearrangement is
(4n +2) electron, then it is thermally suprafacial and photochemically
antrafacial through the aromatic transition state.](https://image.slidesharecdn.com/pericyclicreactions-180921085207/85/Pericyclic-reactions-66-320.jpg)