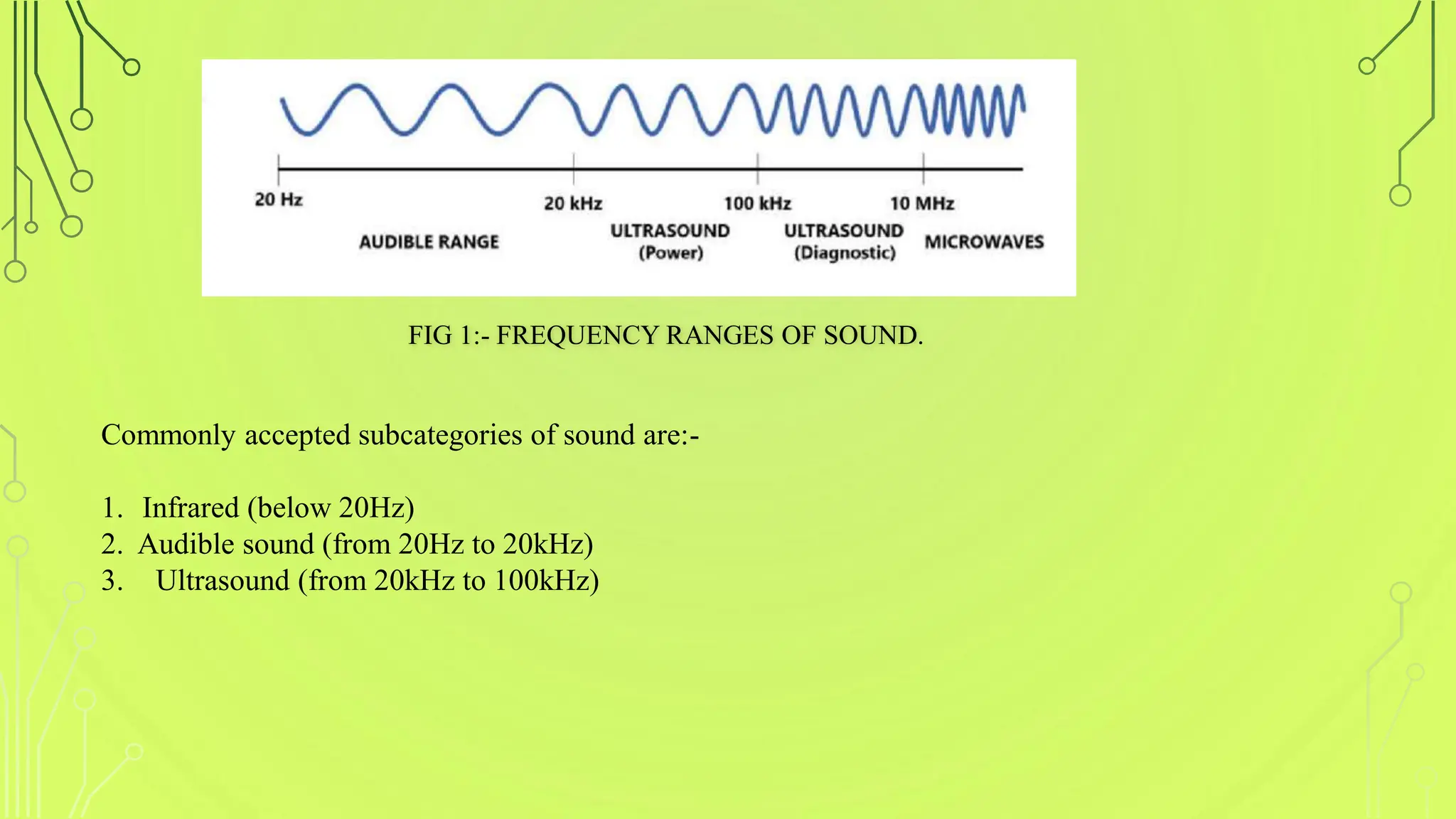
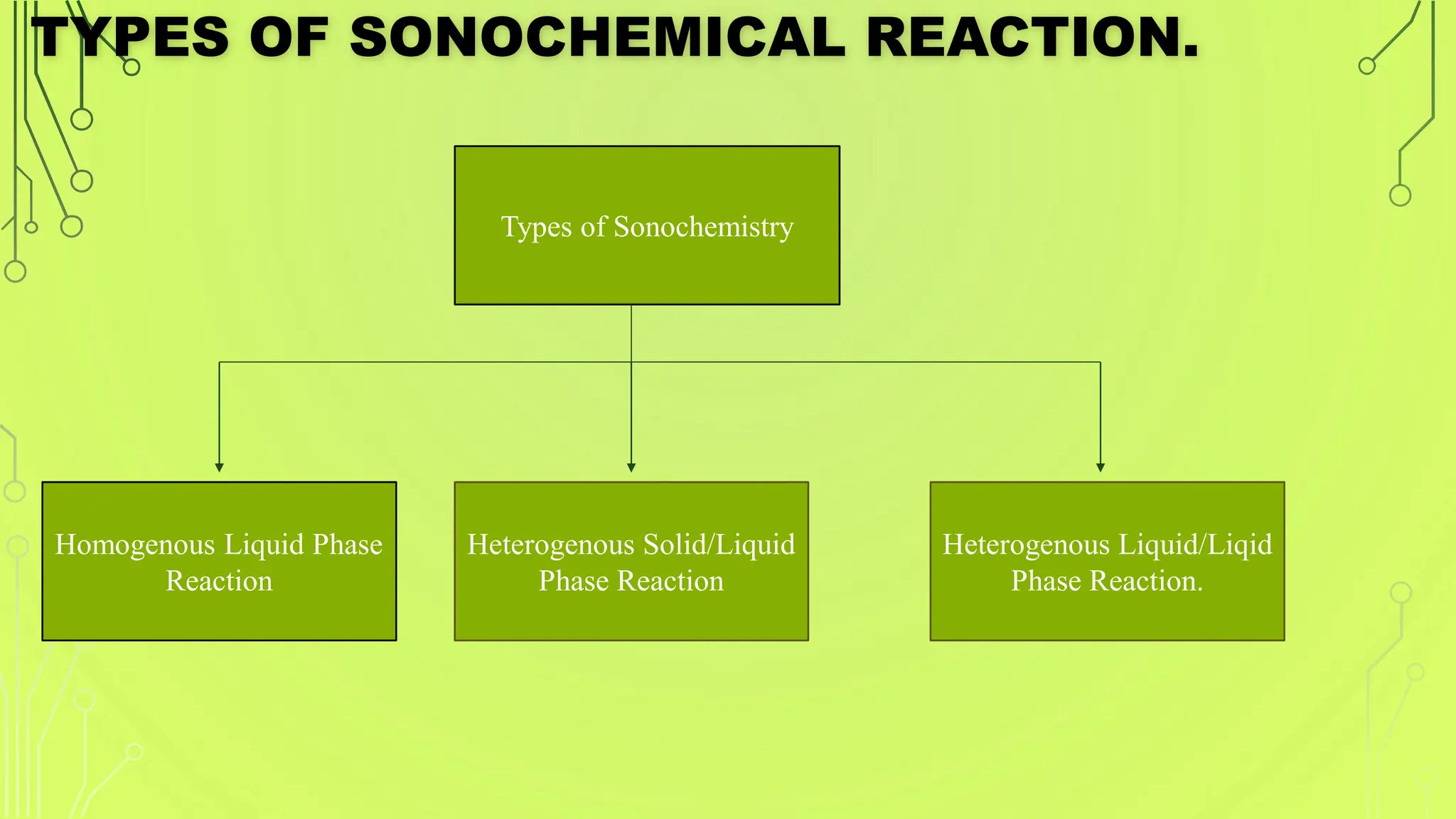
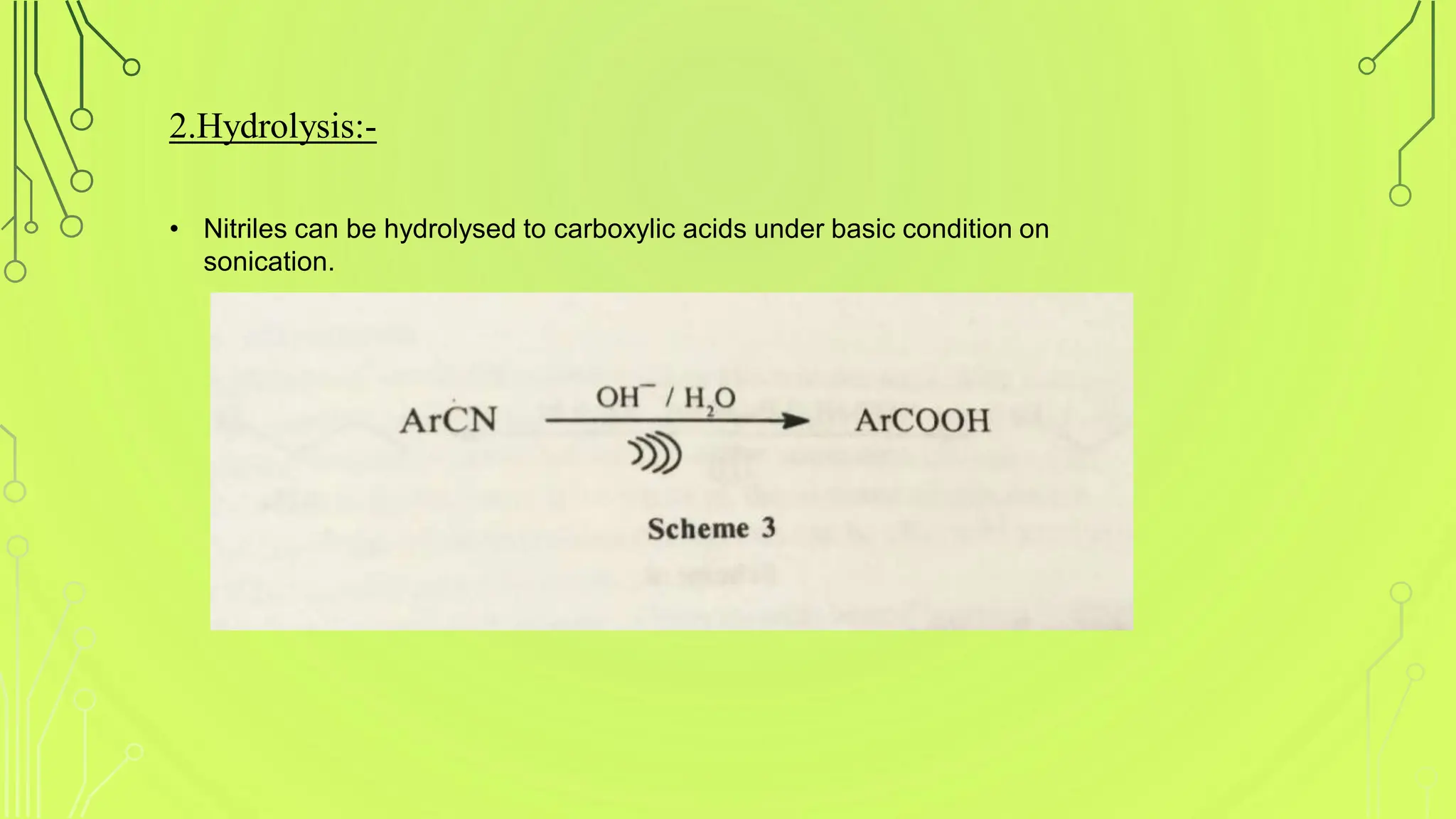
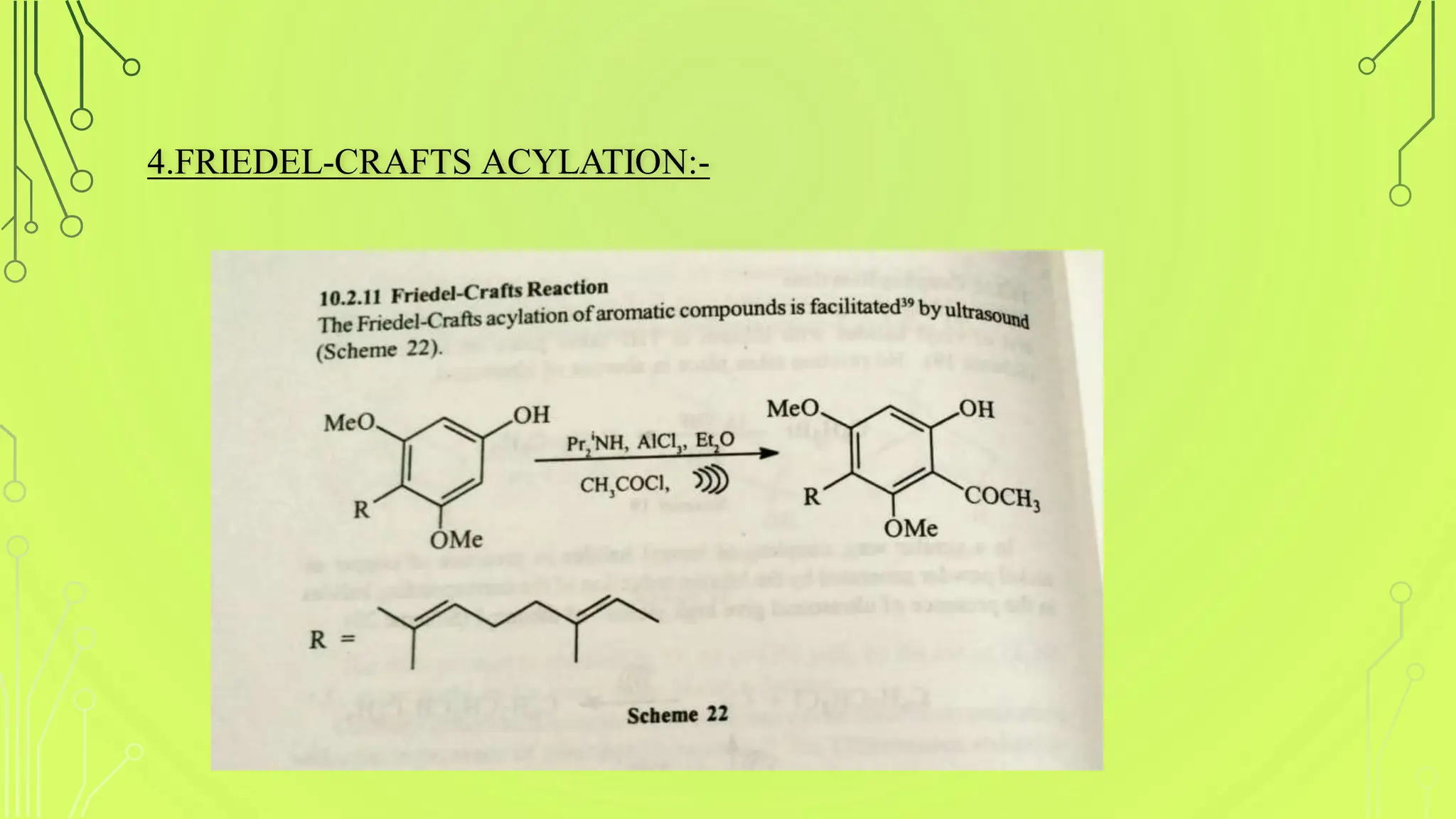
The document discusses ultrasound-assisted reactions, focusing on sonochemistry, which examines the effects of ultrasound on chemical activity. It describes the mechanism of acoustic cavitation, types of reactions such as homogeneous and heterogeneous, and various synthetic applications, highlighting the advantages of sonochemistry including increased efficiency and environmental friendliness. The paper also references notable literature and advancements in the field.