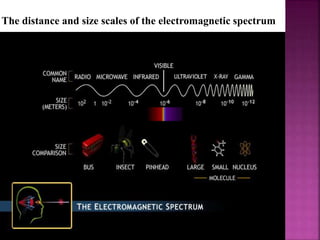
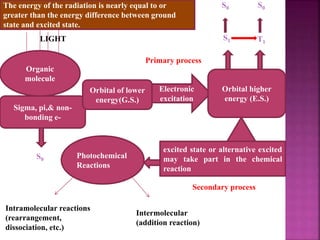
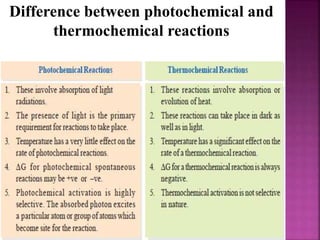
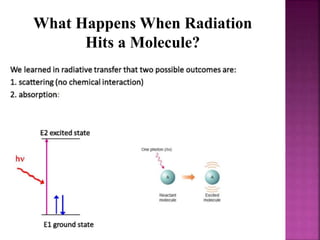
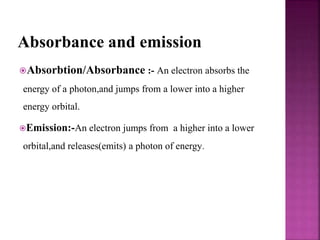
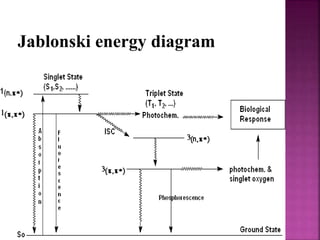
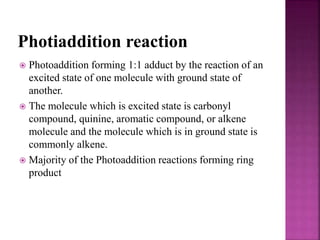
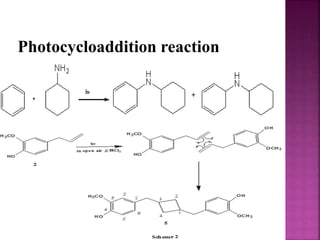
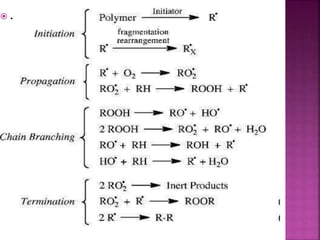
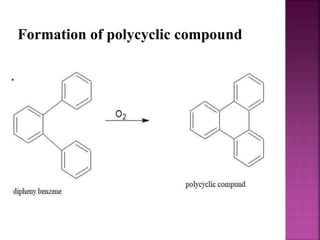
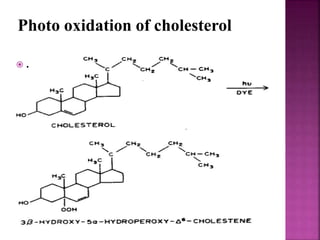
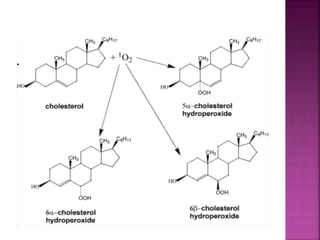
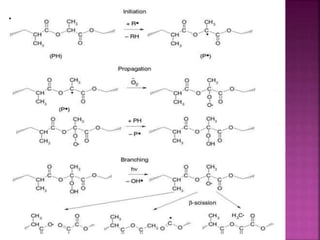
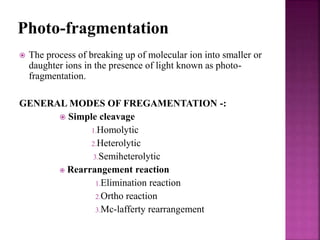
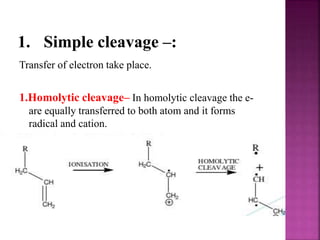
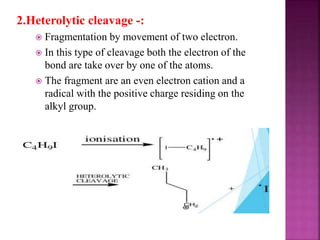
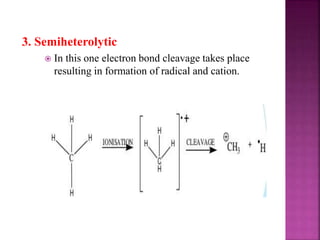
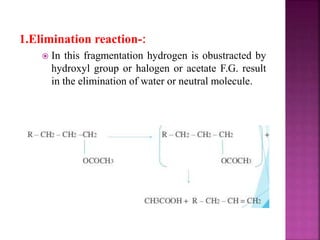
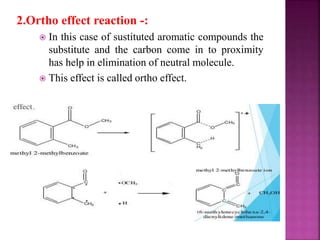
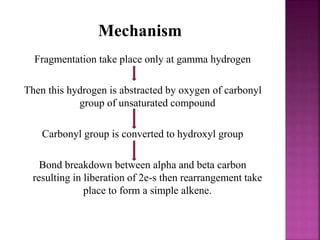
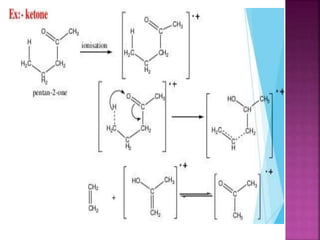
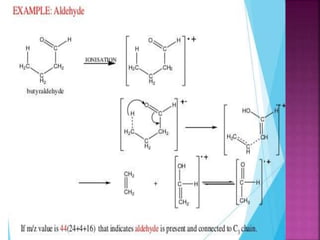
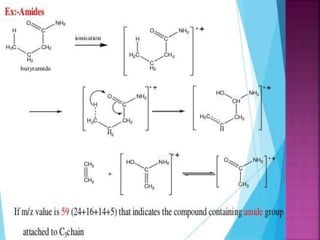
Photochemical reactions are chemical reactions initiated by the absorption of light energy. These reactions involve an organic molecule absorbing a photon which causes electronic excitation from a lower to a higher orbital. The excited molecule may then undergo various chemical reactions, including photoaddition, photocycloaddition, and photo-oxidation reactions. Photochemical reactions differ from thermochemical reactions in their requirement of light to initiate the reaction.