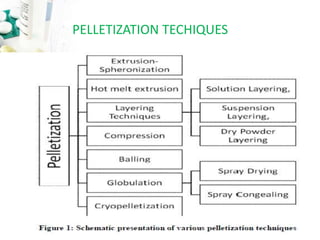
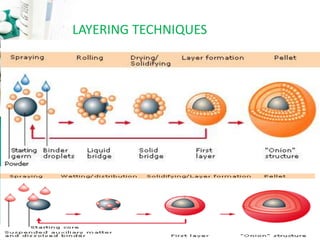
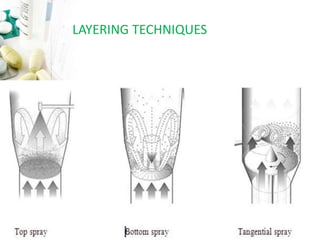
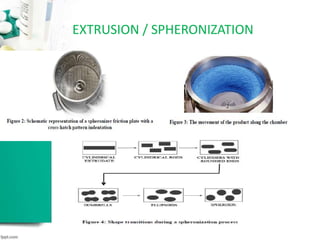
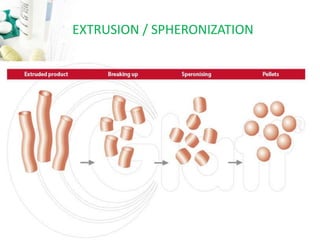
The document discusses pelletization technology, which transforms fine powders into spherical units called pellets, highlighting its advantages such as dose uniformity and flow properties, as well as disadvantages including cost and complexity. It covers various pelletization techniques including layering, extrusion, and spheronization, detailing process variables and equipment considerations. Additionally, it outlines pellet characterization parameters essential for evaluating quality.