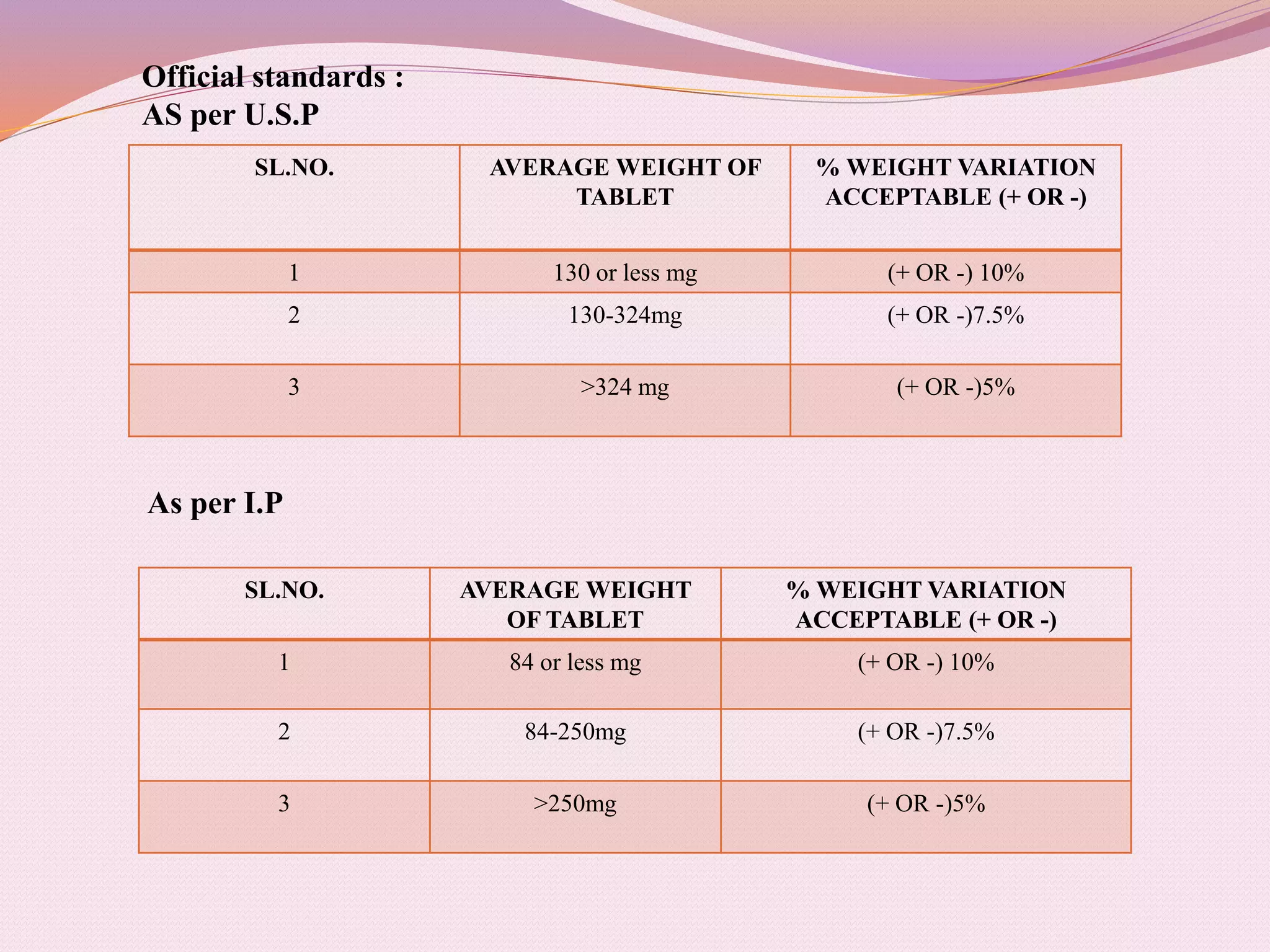
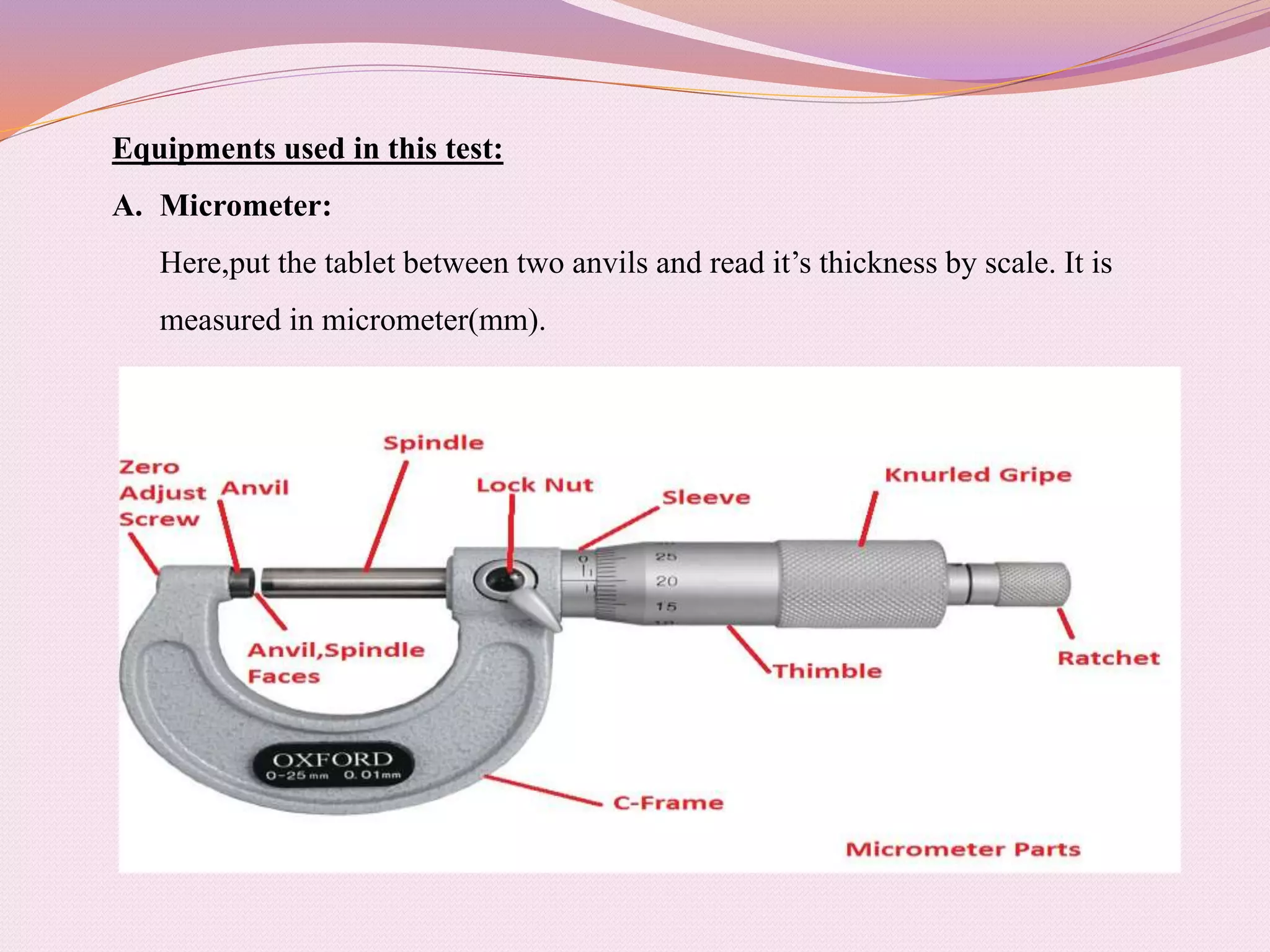
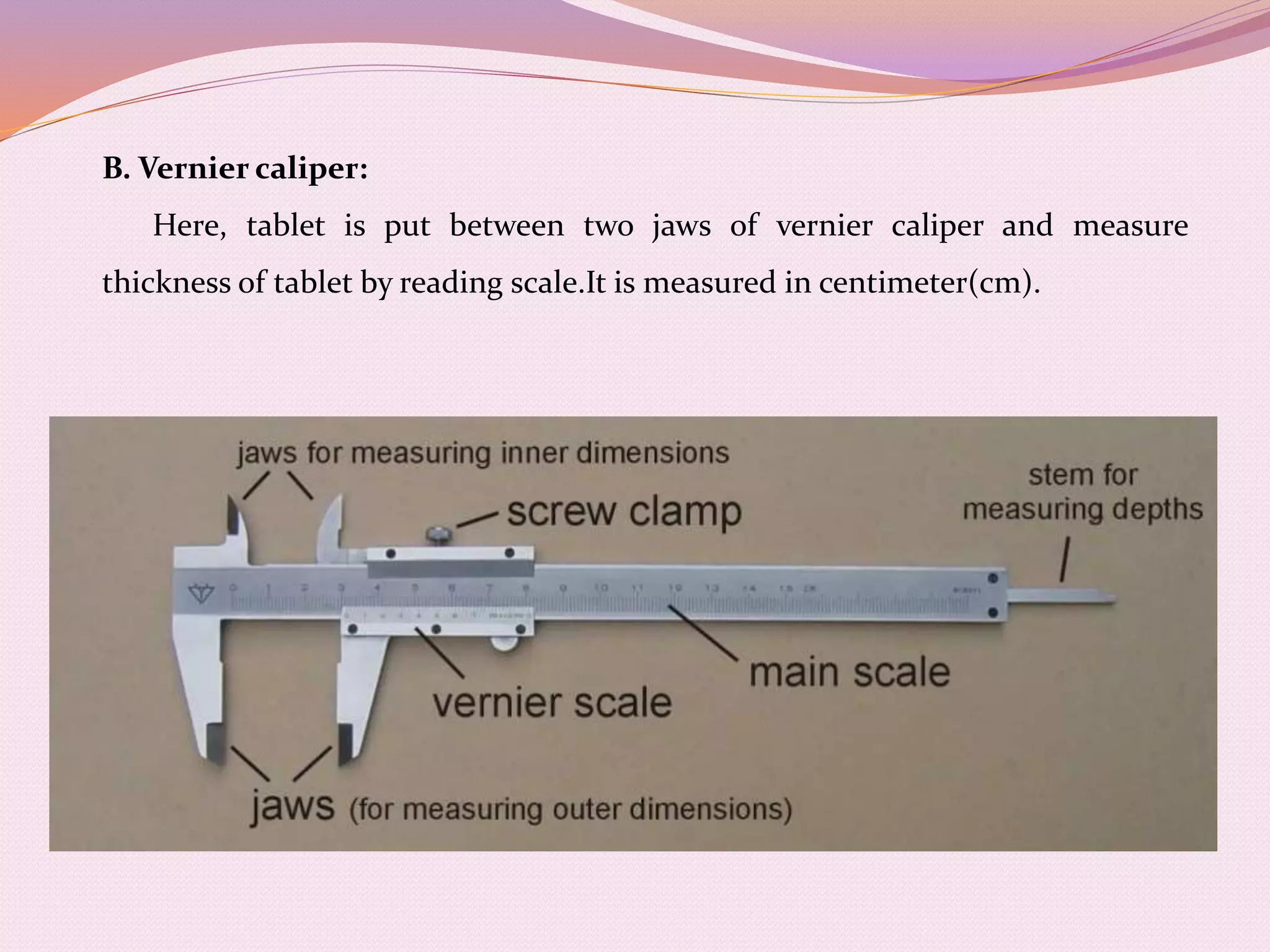
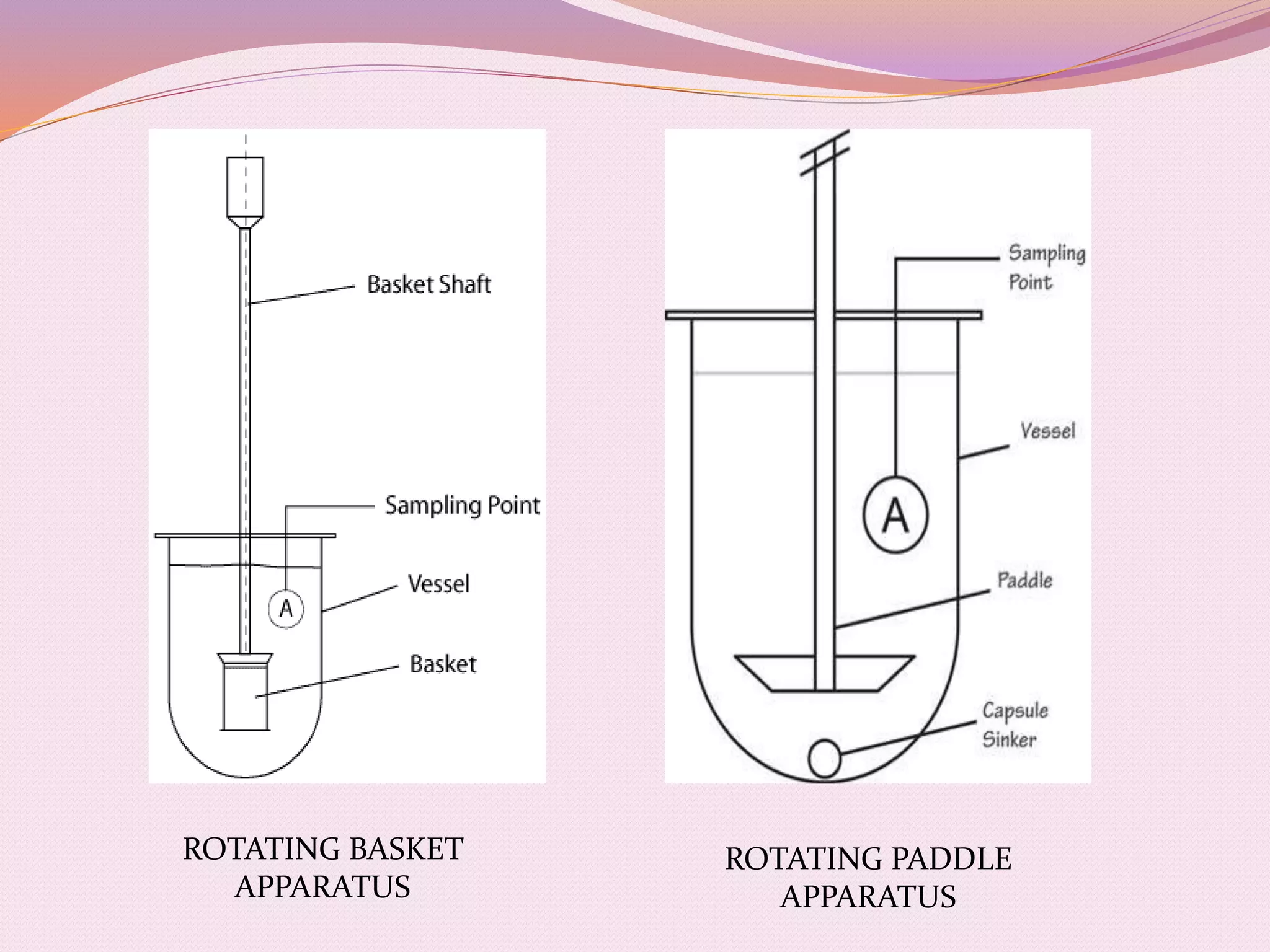
The document provides information on quality control testing for pharmaceutical tablets. It defines quality control as the process of monitoring quality during manufacturing to ensure standards are met. It describes several important quality control tests conducted on tablets, including weight variation, thickness, hardness, friability, disintegration, dissolution, and content uniformity tests. These tests are essential to ensure tablets are safe, effective, and meet specifications for attributes like drug content, stability and patient acceptability. The document provides details on procedures, equipment and acceptance criteria for each quality control test.