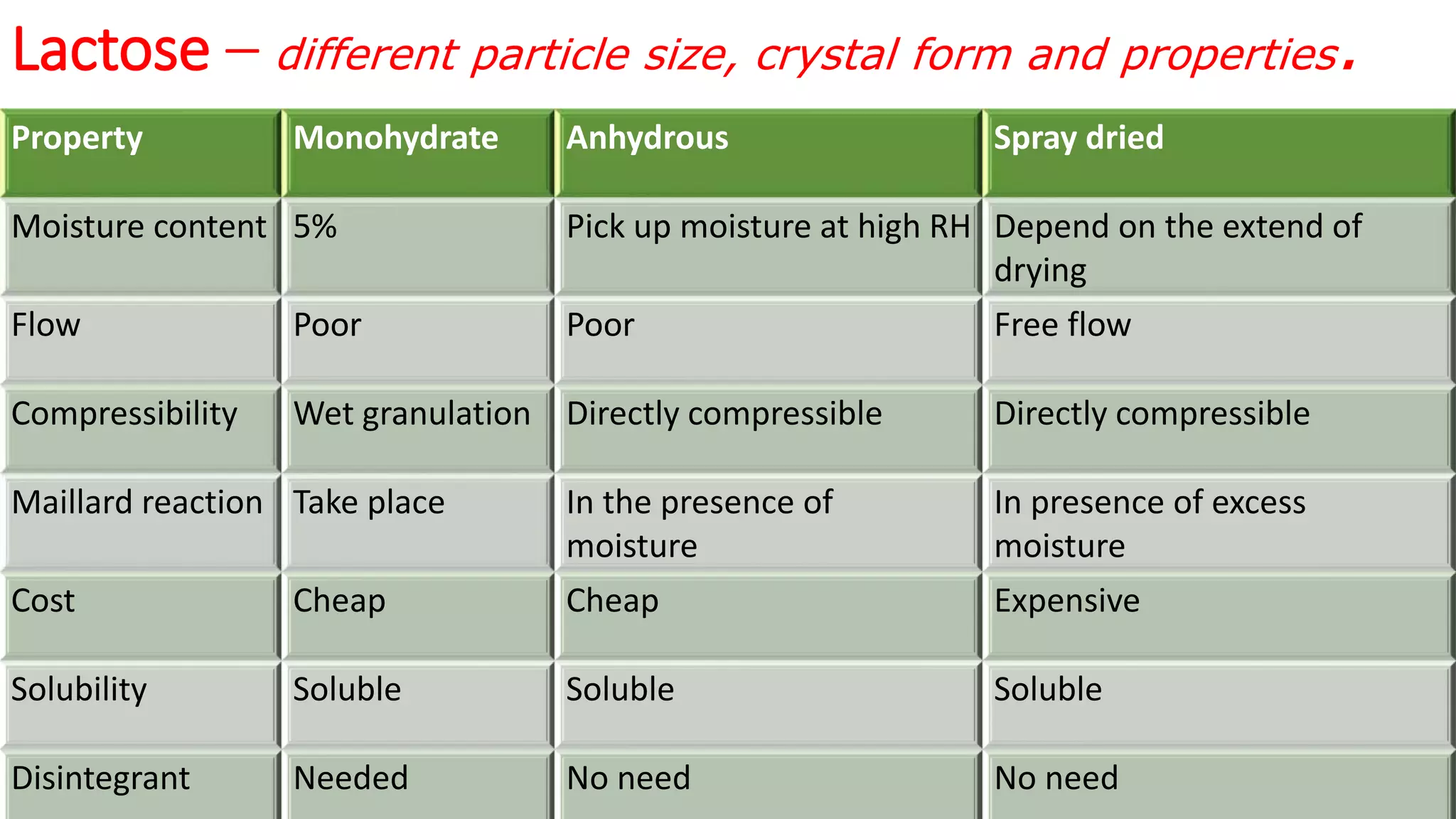
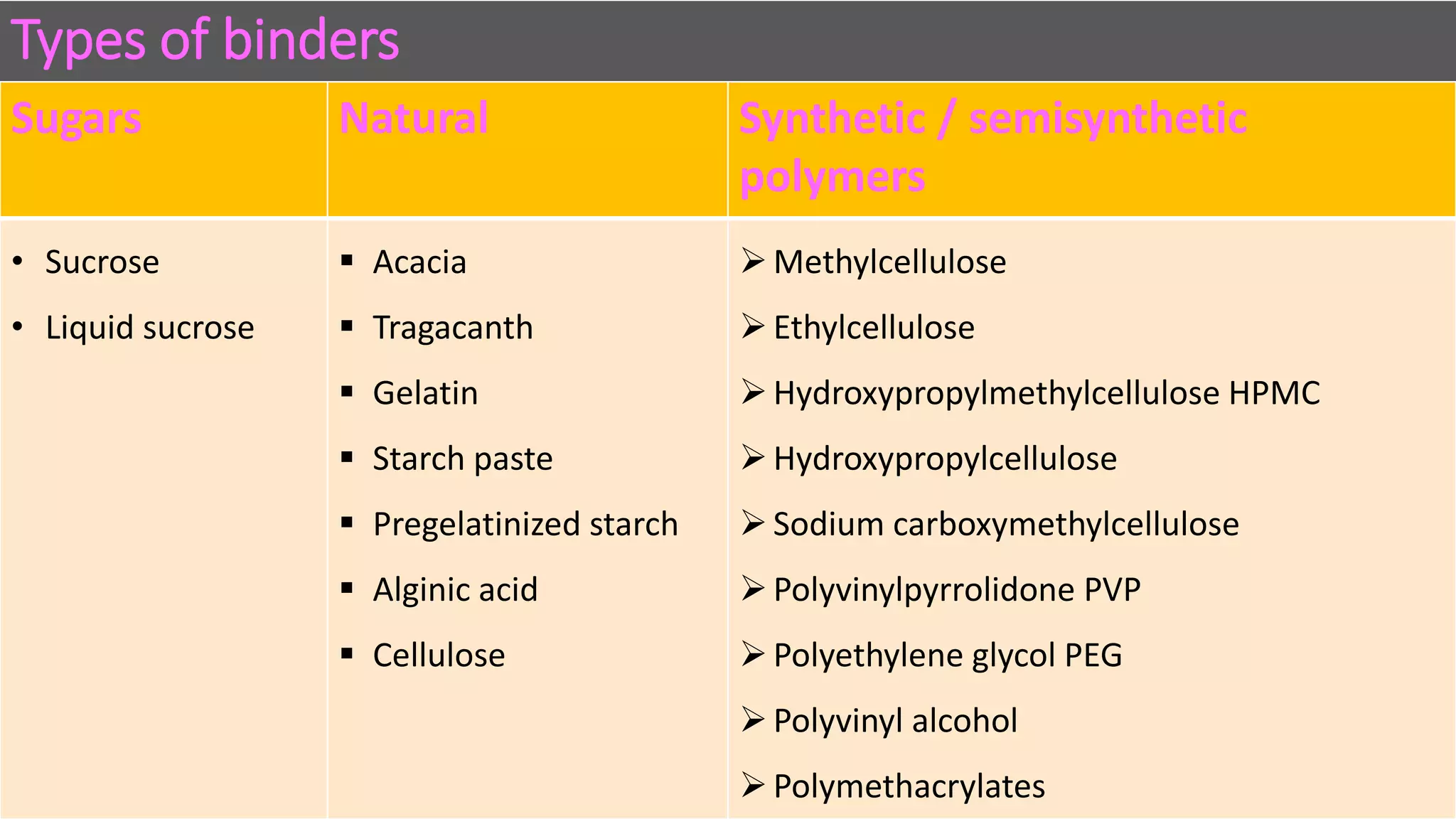
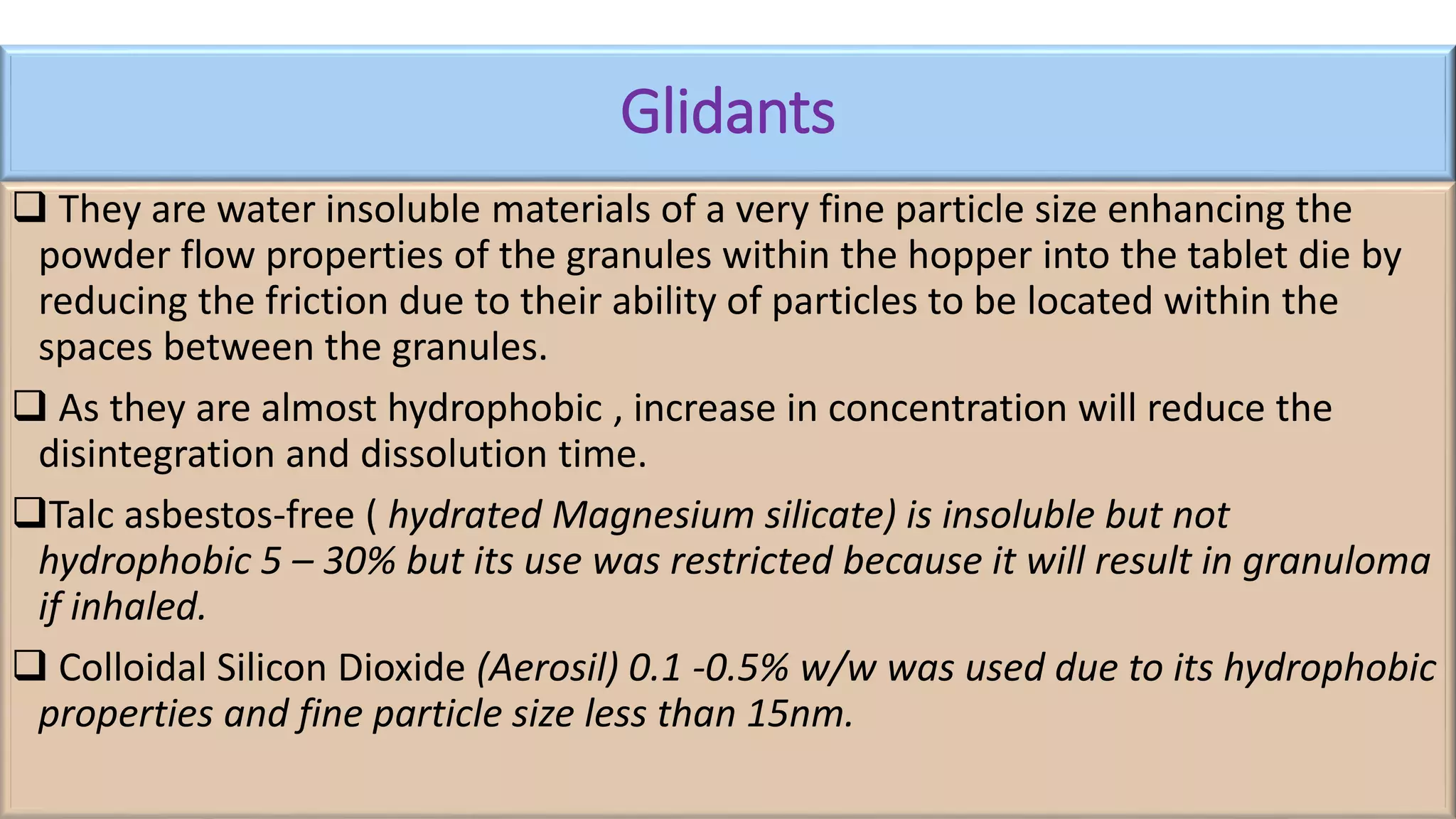
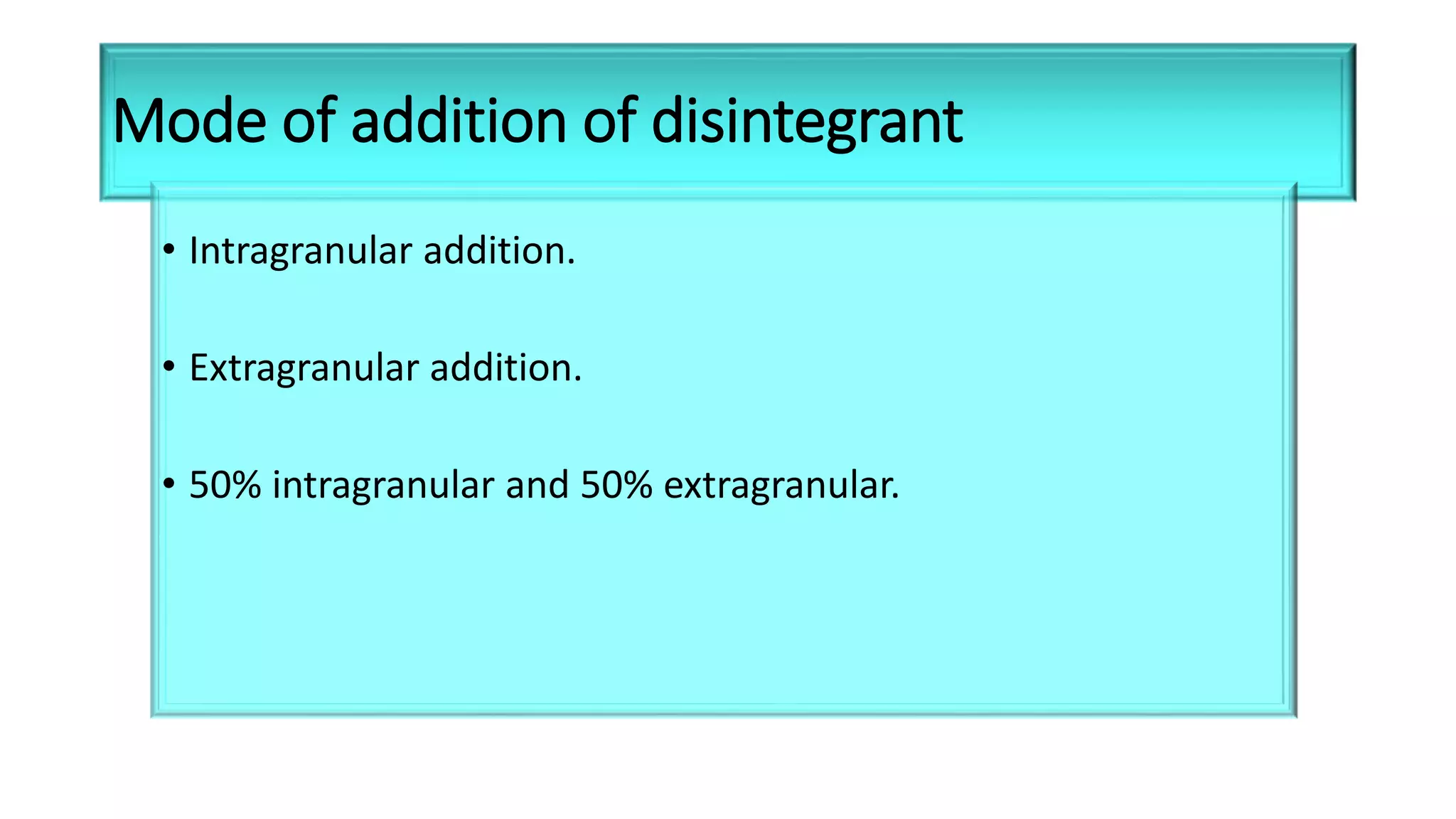
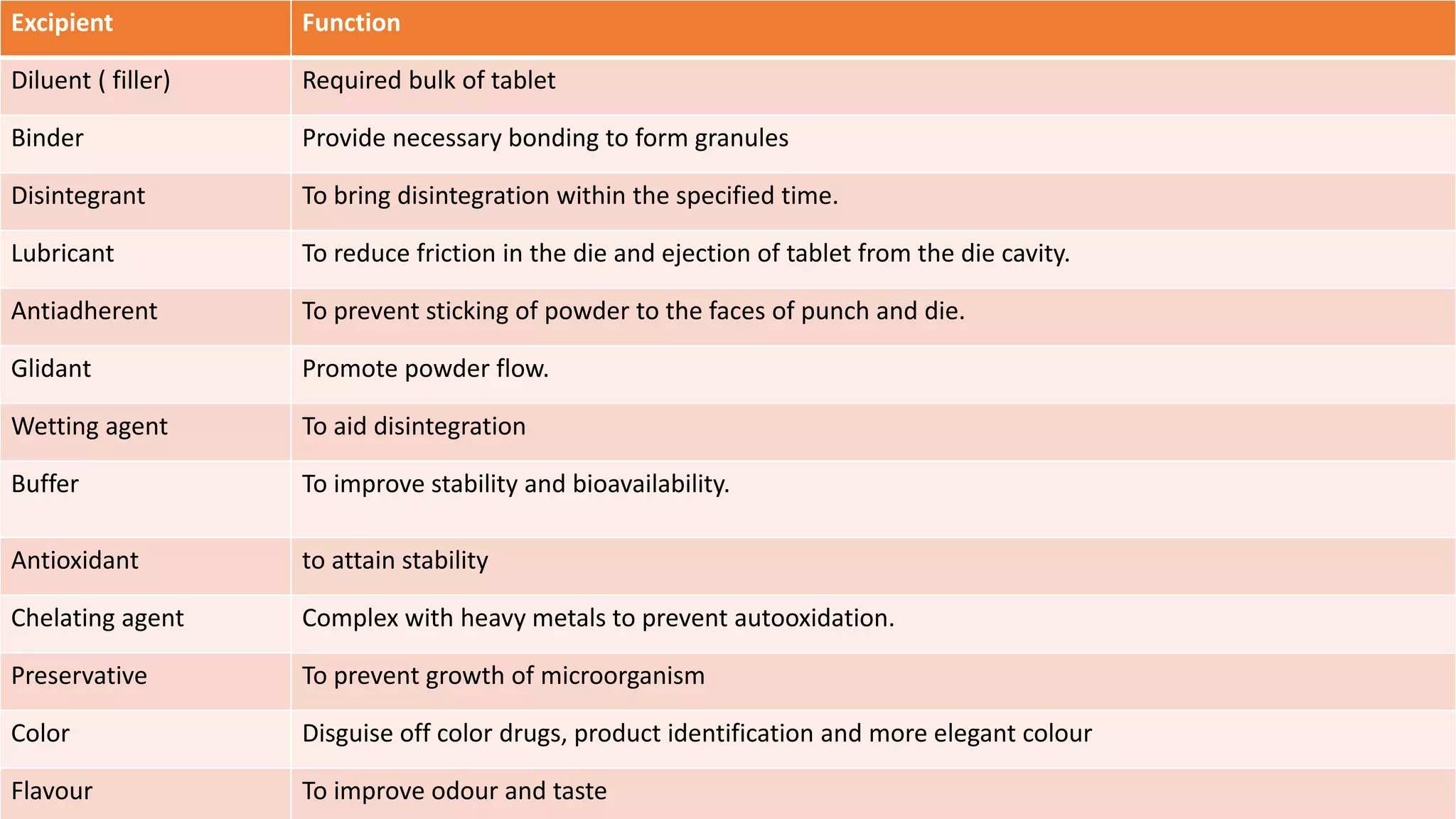
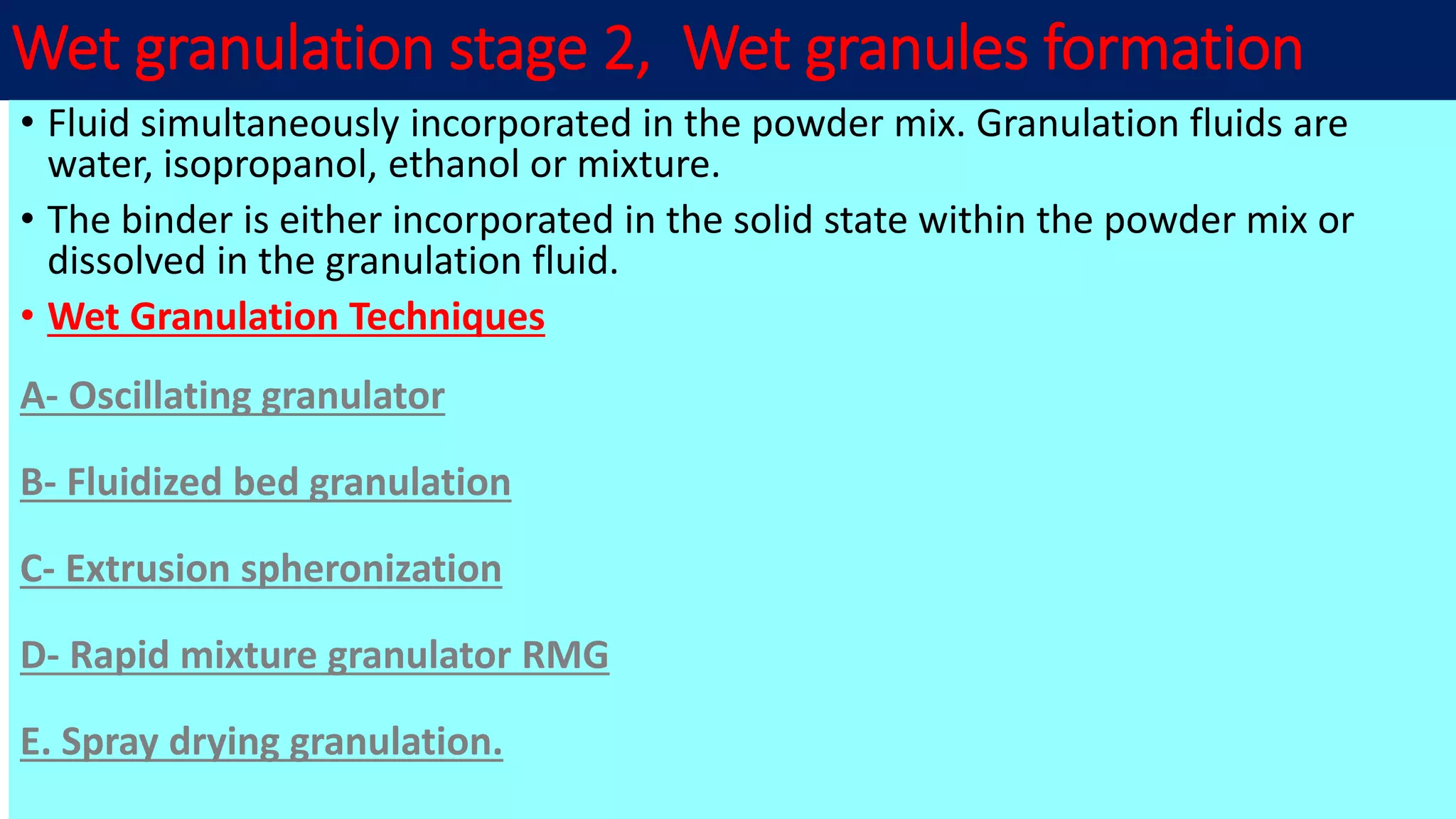
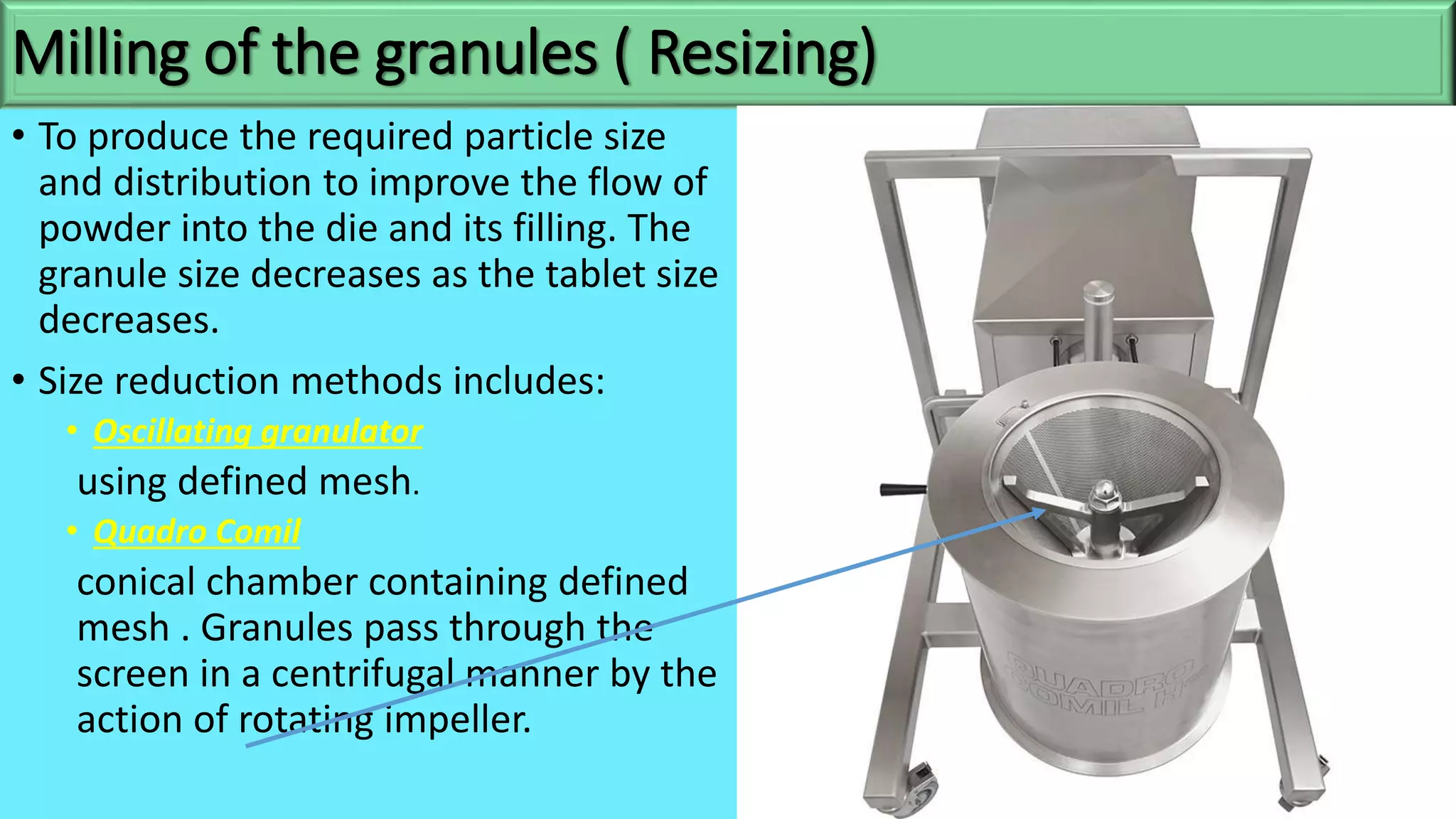
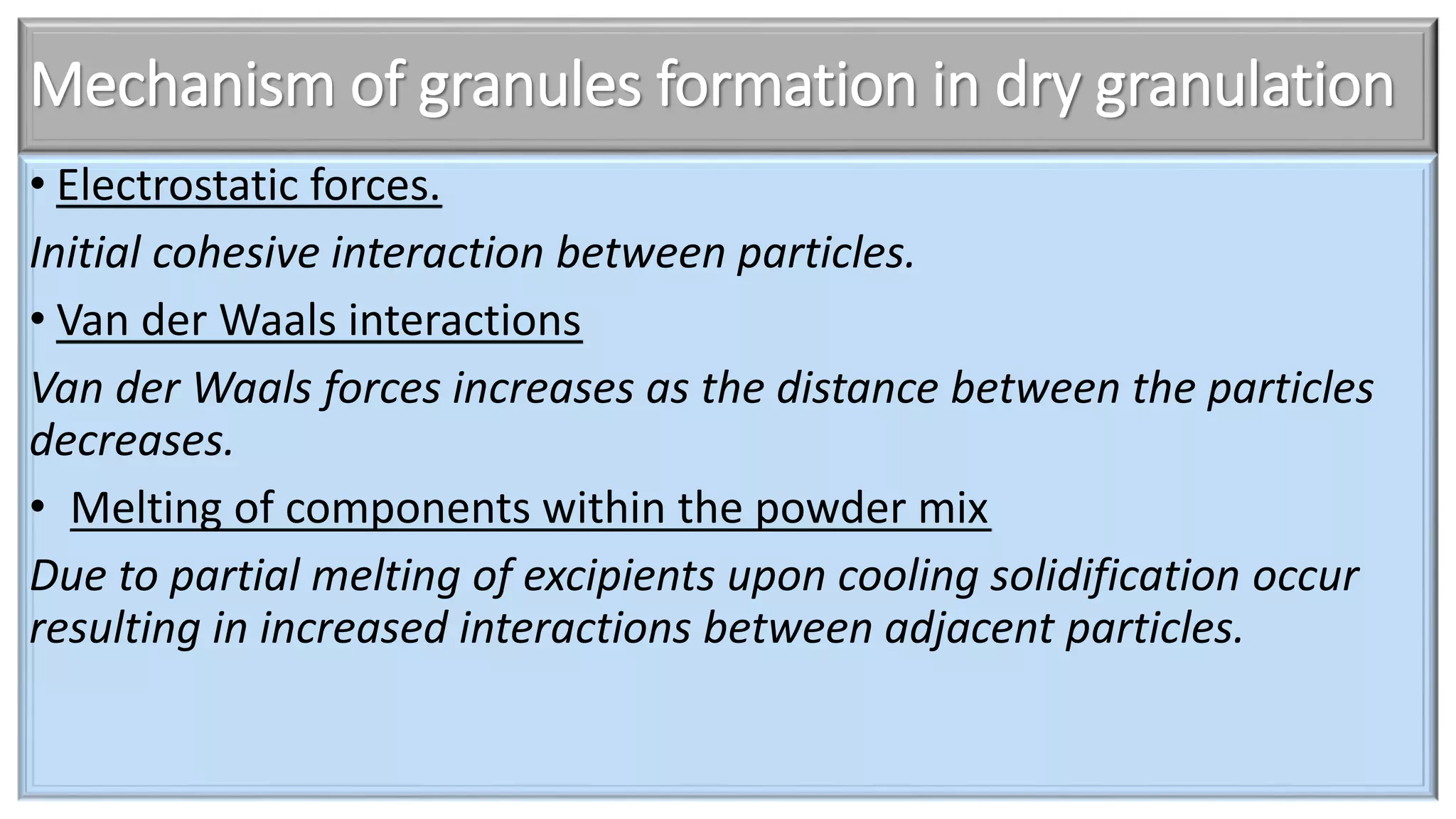
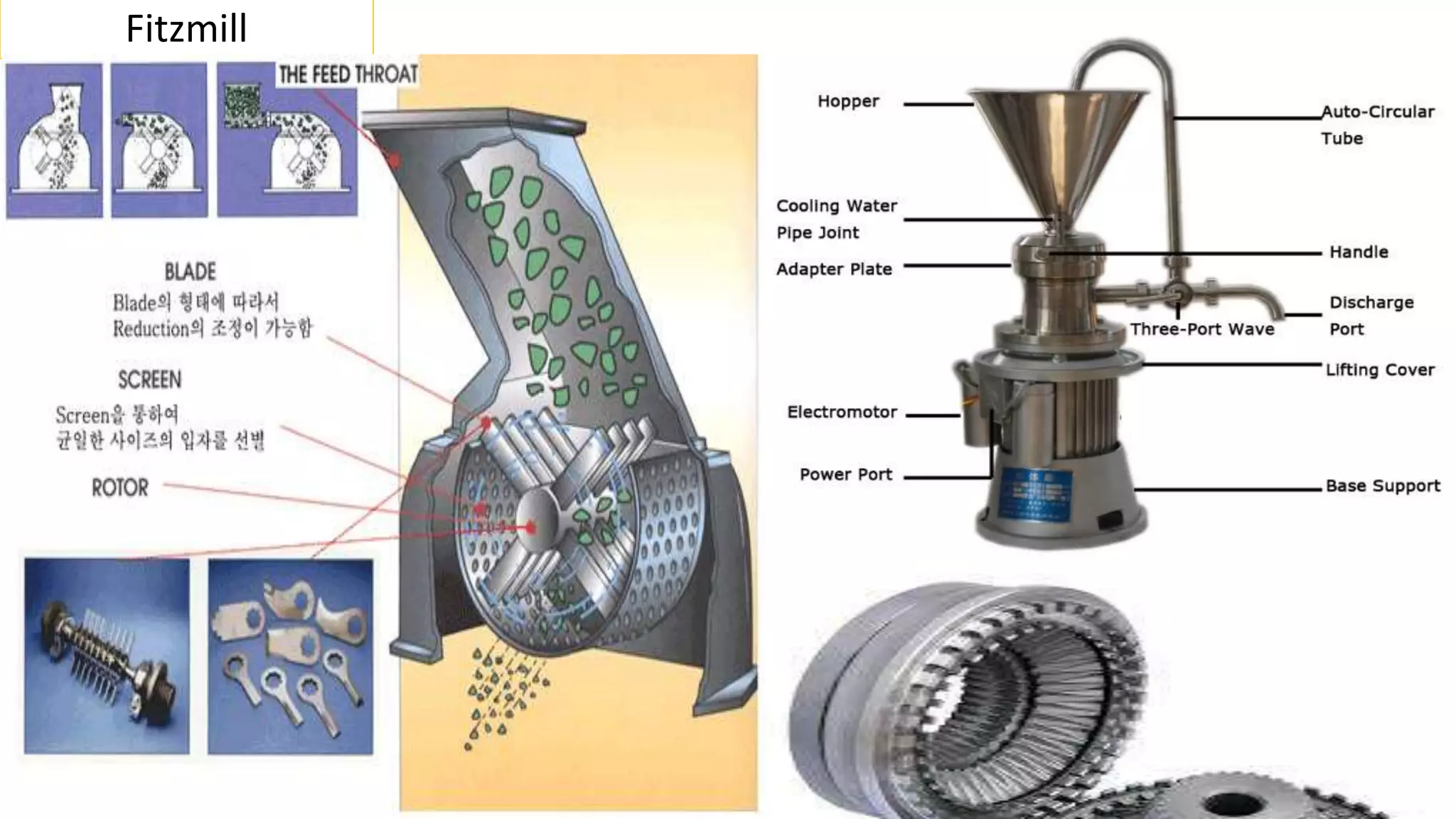
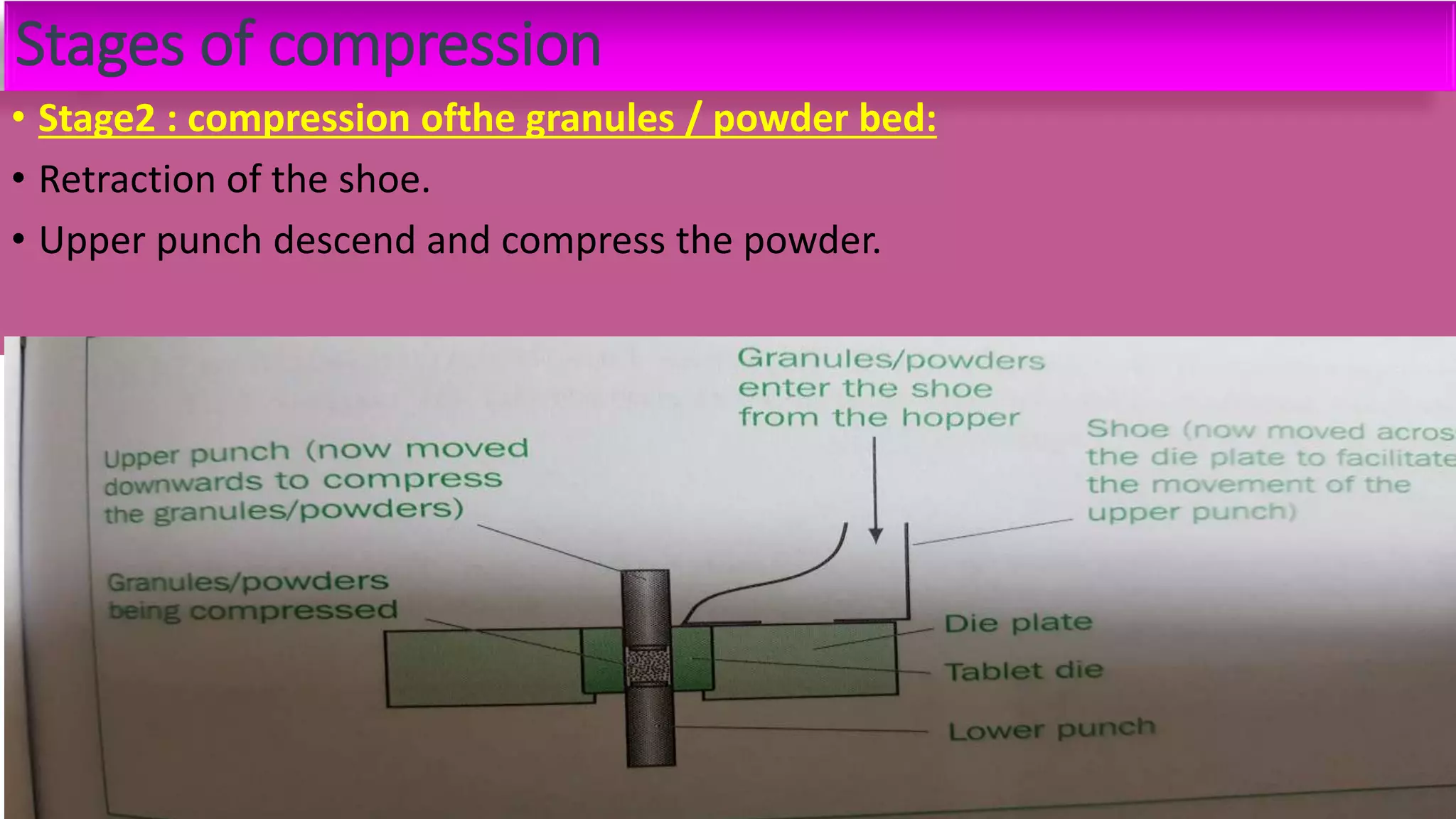
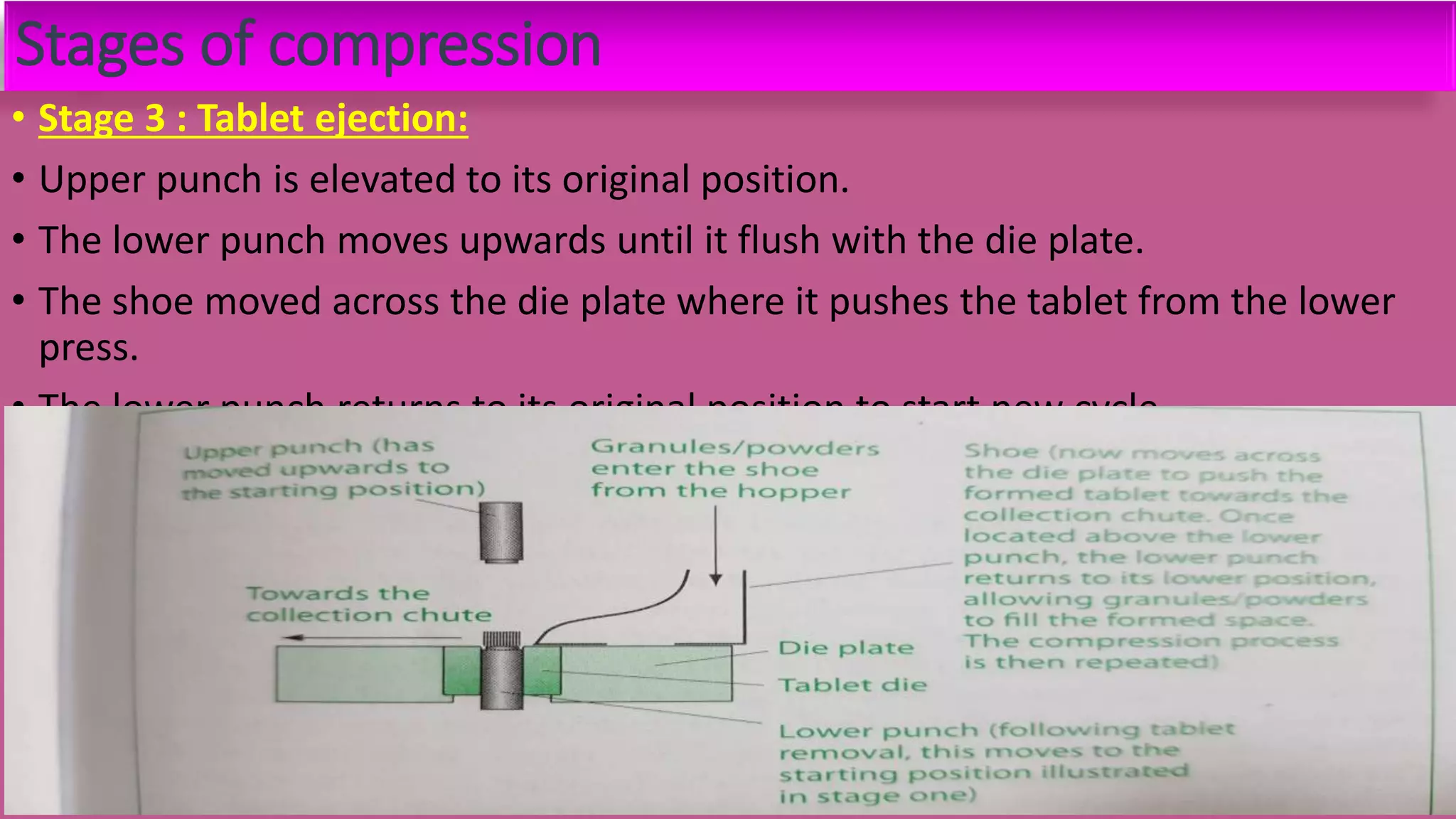
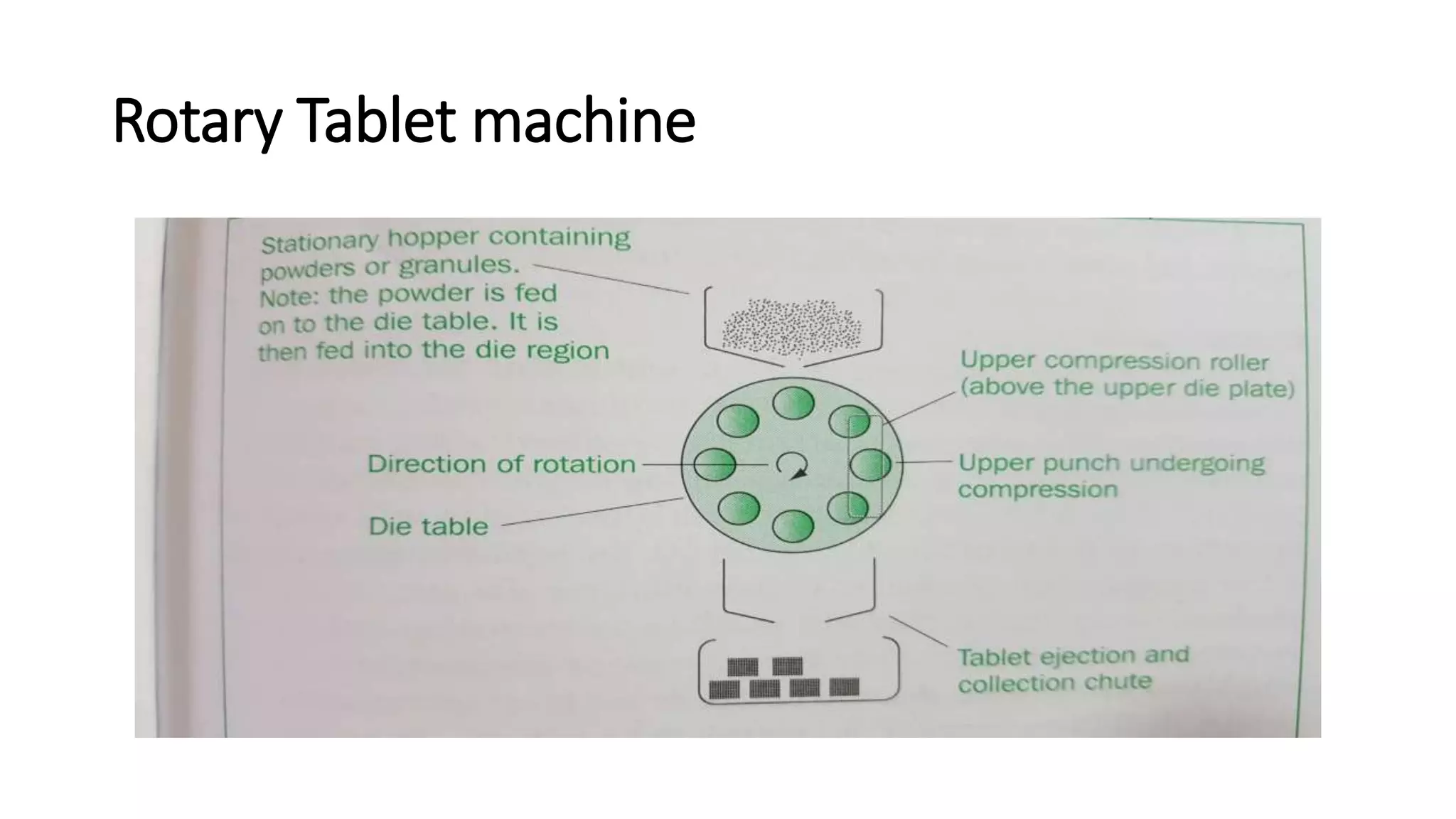
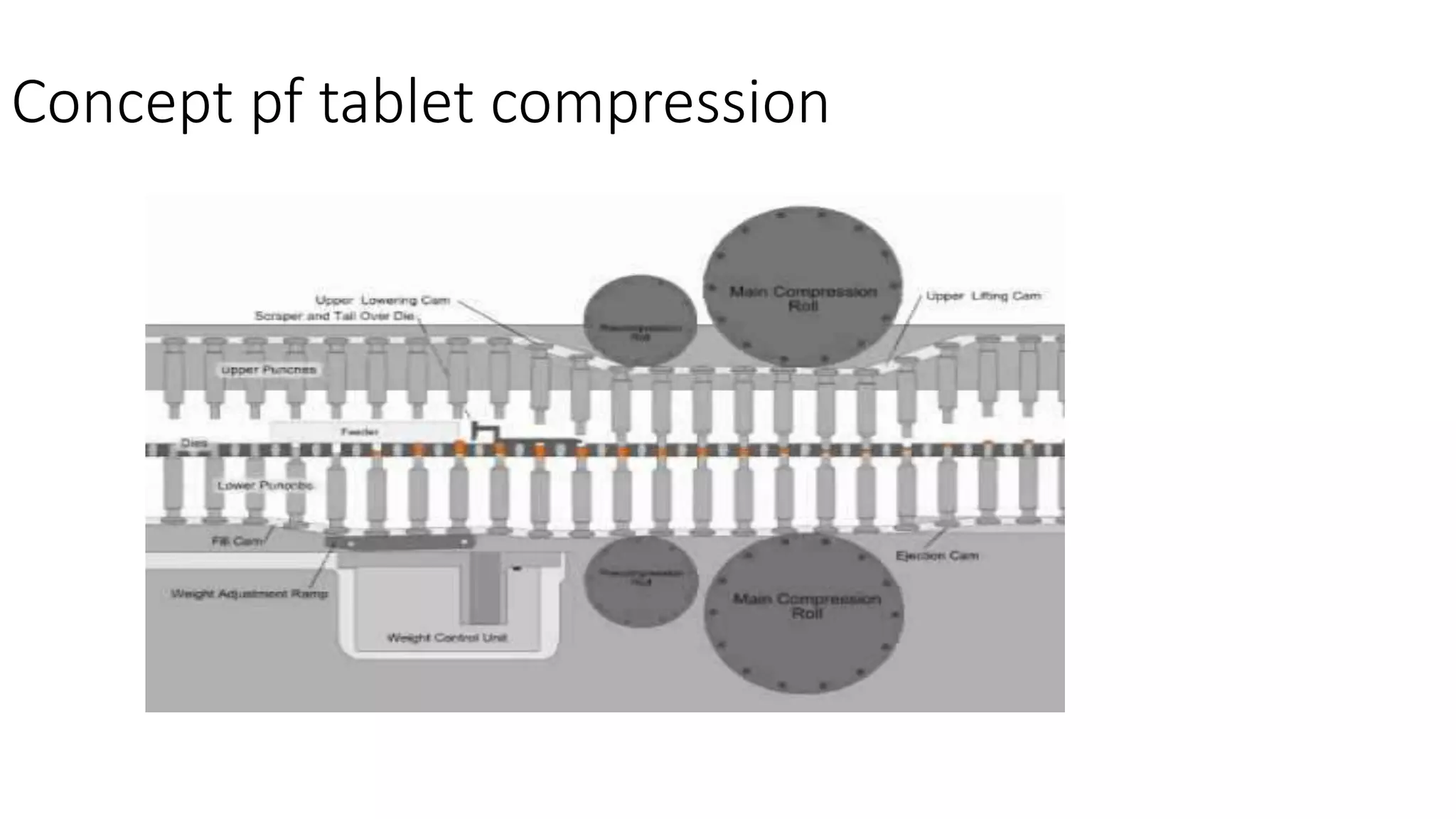
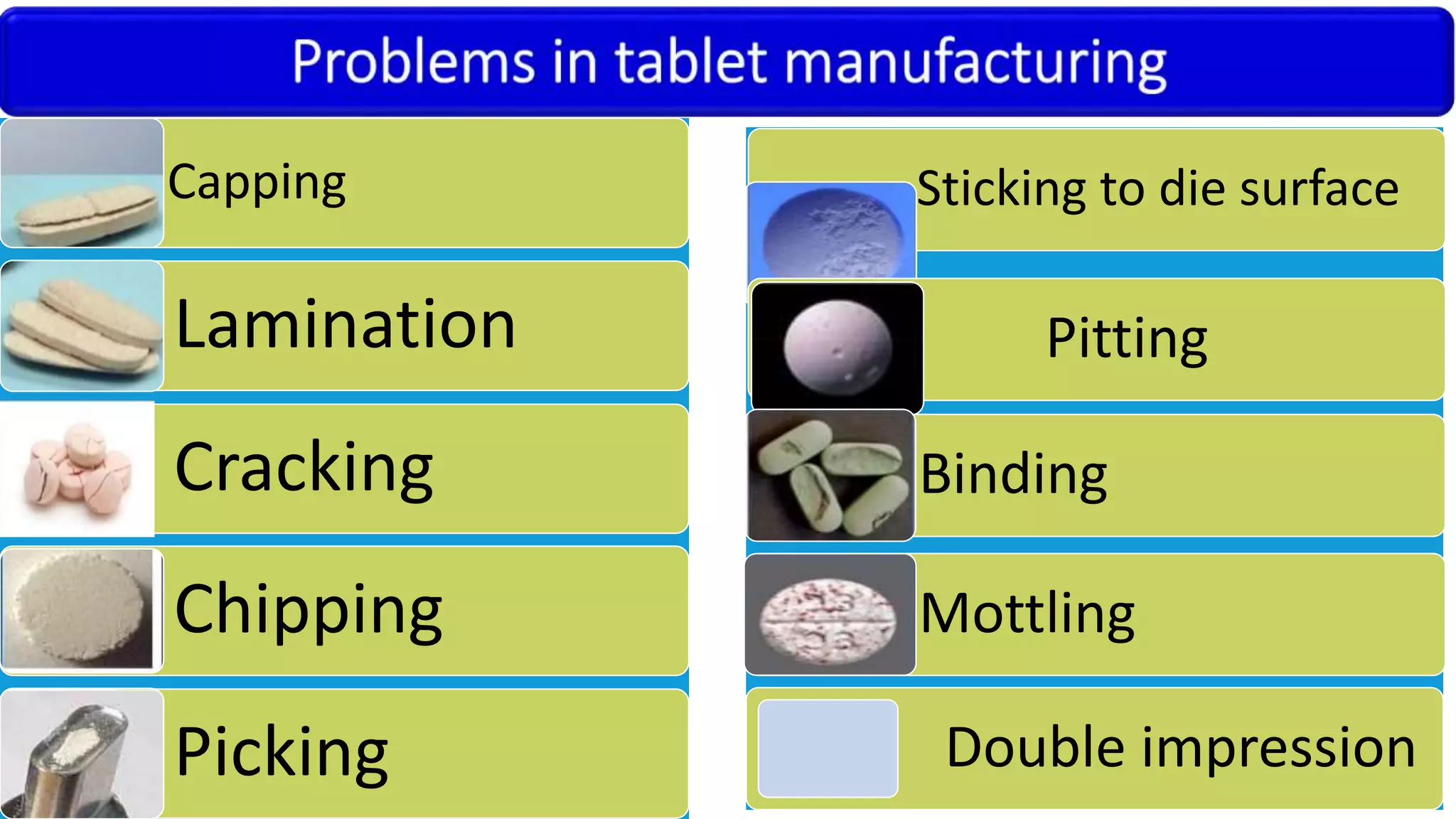
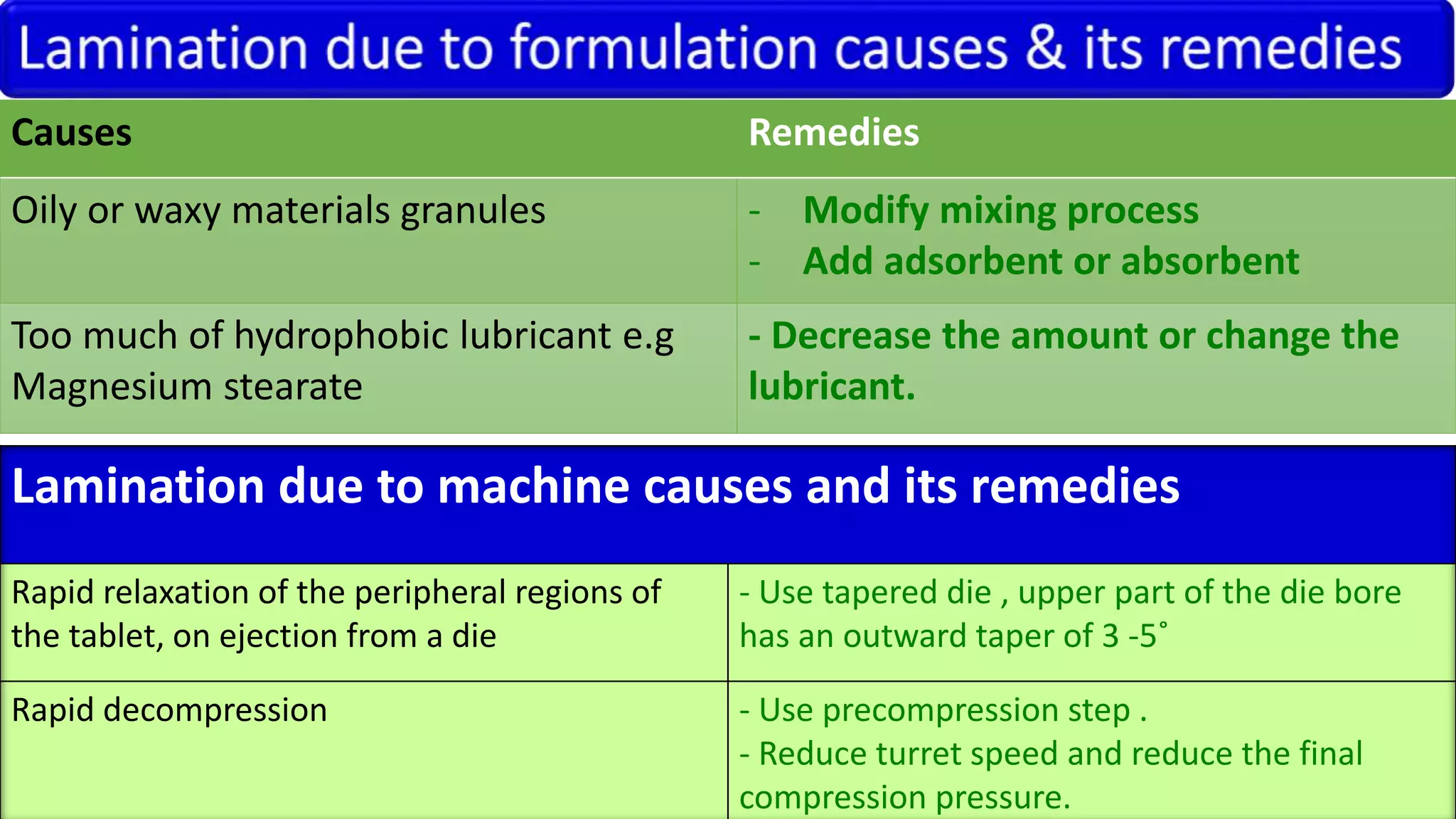
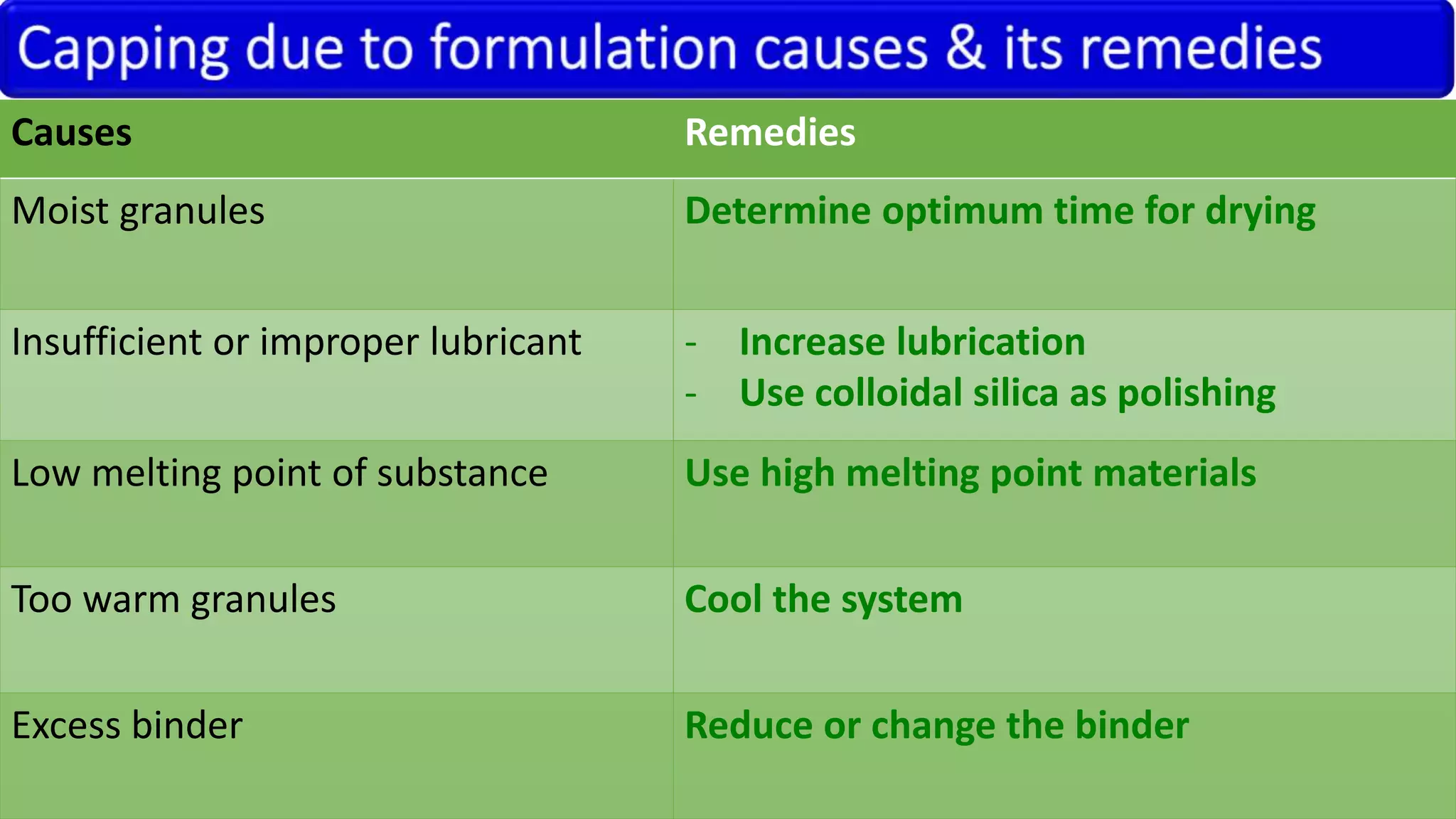
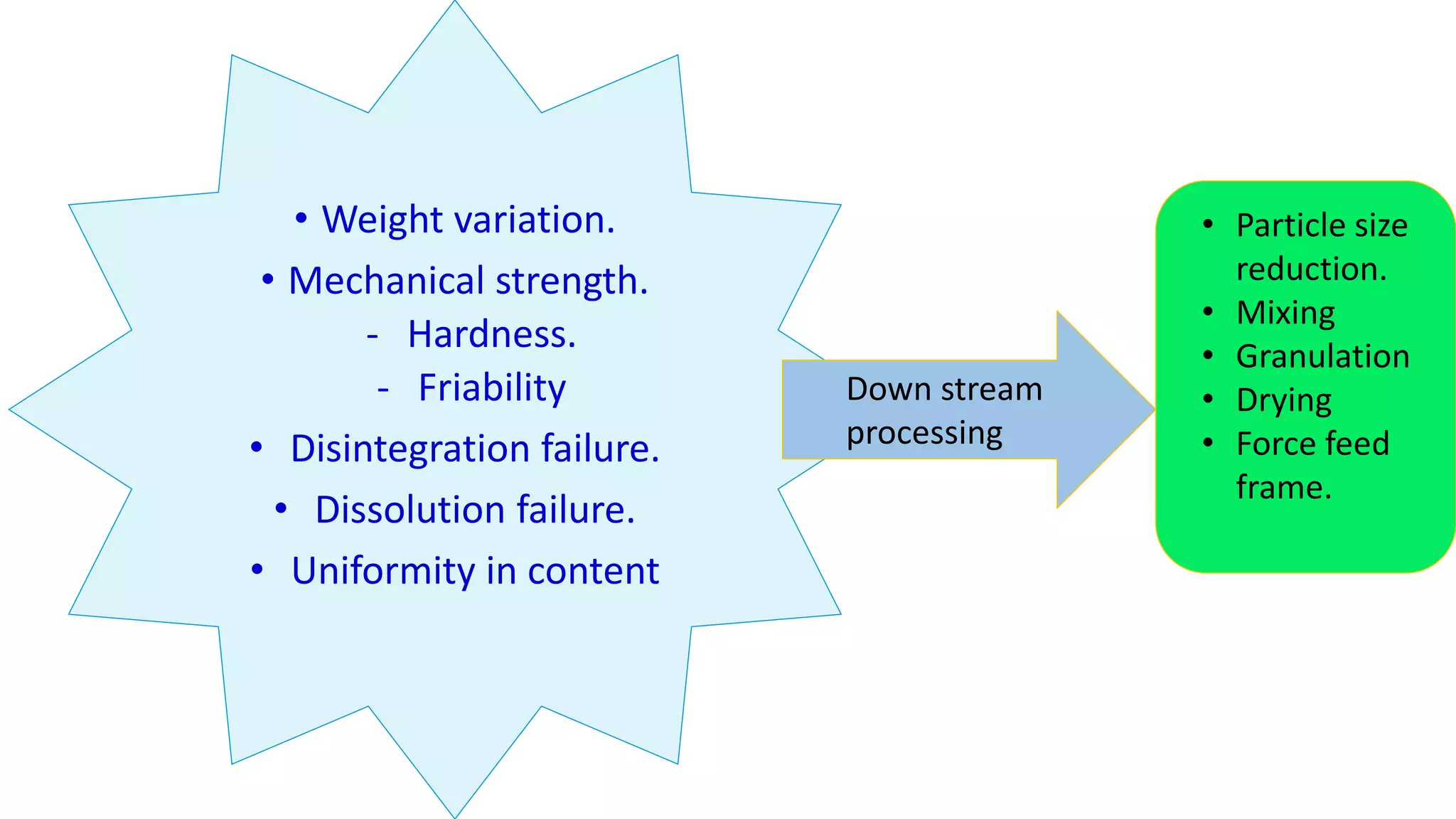
This document discusses pharmaceutical preparations and tablets. It defines pharmaceutical preparations as medicinal products consisting of active substances that may be combined with excipients and formulated into a suitable dosage form. The document outlines different types of pharmaceutical preparations including licensed and unlicensed preparations. It also discusses production requirements and testing. The document focuses on tablets, defining them and outlining different tablet categories. It discusses characteristics, advantages, and disadvantages of tablets. The document covers desired properties of active pharmaceutical ingredients and excipients used in tablet formulations. It provides details on commonly used excipients like diluents, binders, lubricants, and their functions in tablet formulations.