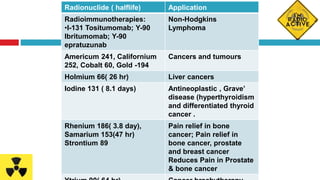
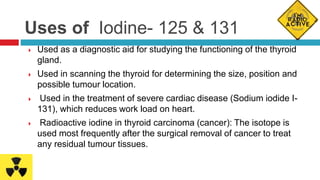
Radiopharmaceuticals are radioactive pharmaceutical agents used for diagnostic or therapeutic procedures. They consist of a carrier molecule and a radionuclide. Common isotopes used include iodine-131, technetium-99m, cobalt-57, cobalt-60, gold-198, and iodine-125. Radiopharmaceuticals allow non-invasive monitoring of biological processes through imaging techniques. They are produced in specialized facilities and handled with precautions due to their radioactivity, requiring shielding and controlled storage conditions. Clinical applications include diagnosis of thyroid function and cancer treatment through targeted radiation exposure.







![Radiopharmaceutical
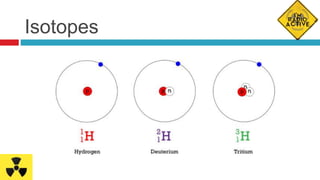
A Radiopharmaceutical is a drug that can be used
either for diagnostic or therapeutic purposes. It is
composed of a radioisotope bond to an organic
molecule. The organic molecule conveys the
radioisotope to specific organs, tissues or cells.
Radioactive element - 133Xe
Labeled compounds - 131I iodinated proteins
99mTc labeled compounds
[18F]FDG](https://image.slidesharecdn.com/radiopharmaceuticallecture-230722055012-0425d878/85/Radio-pharmaceuticals-pptx-14-320.jpg)