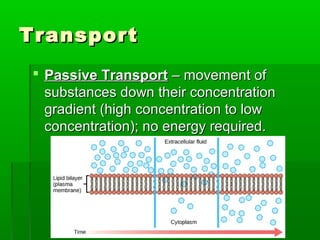
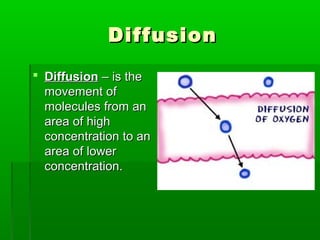
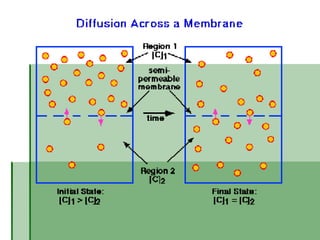
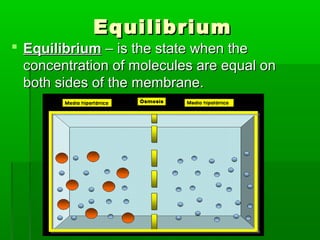
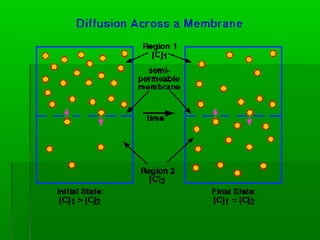
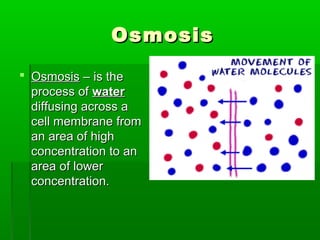
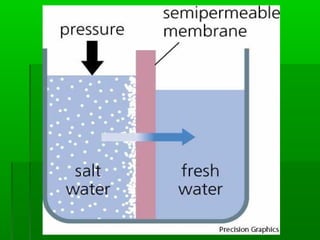
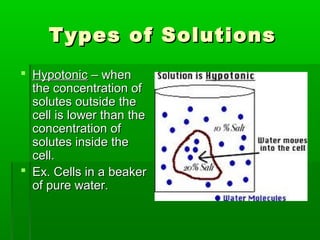
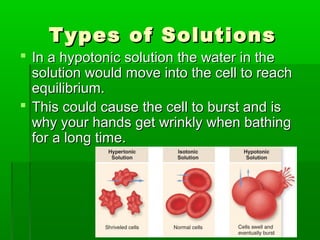
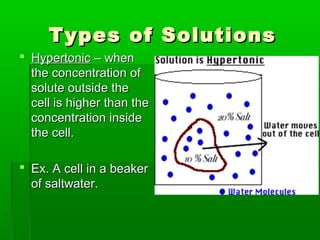
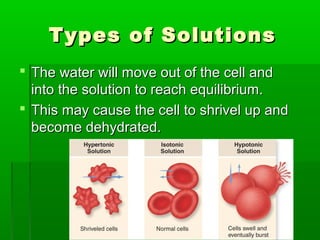
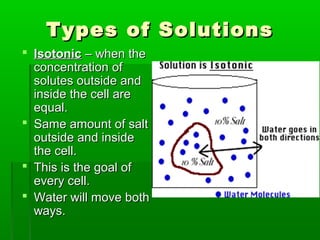
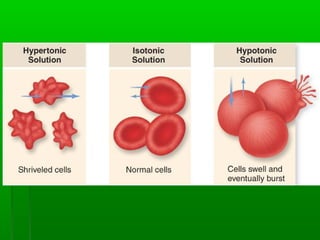
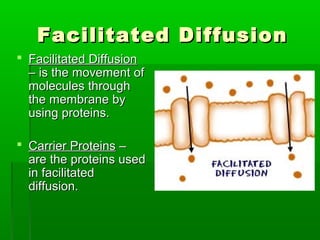
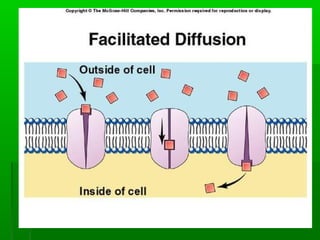
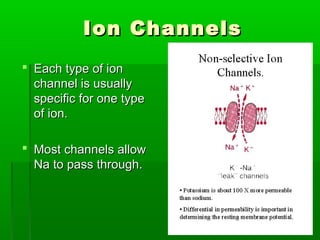
Passive transport involves the movement of substances down their concentration gradient without requiring energy. There are four main types of passive transport: diffusion, osmosis, facilitated diffusion, and diffusion through ion channels. Osmosis is the diffusion of water across a semipermeable membrane from an area of high water concentration to low concentration and does not require energy. When a cell is placed in solutions of different concentrations, water will move in or out of the cell by osmosis to reach equilibrium. [/SUMMARY]