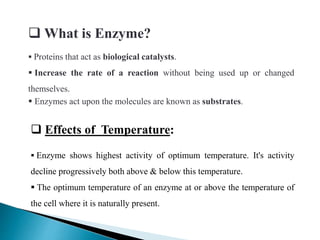
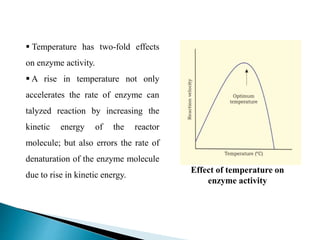
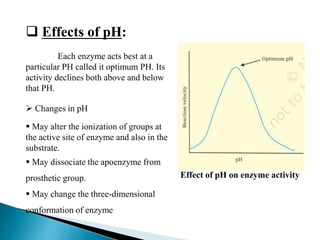
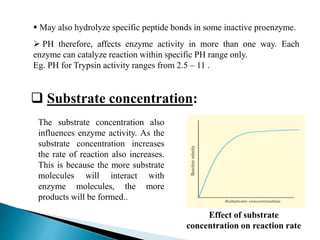
This document discusses enzymes and how various factors affect their activity. It explains that enzymes are proteins that act as biological catalysts, and that their activity is influenced by temperature, pH, substrate and enzyme concentration. The optimum temperature and pH are described as well as how rises or falls from these can impact activity. Isoenzymes, which are variants of the same enzyme found in different tissues, are also covered. The concept of rate-limiting enzymes, which determine the overall rate of a metabolic pathway, is defined.