Embed presentation
Download to read offline


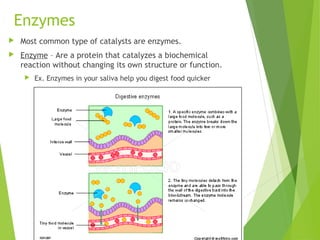
Cells use catalysts to speed up chemical reactions by reducing the activation energy required. Catalysts work by lowering the energy barrier of a reaction without being used up in the process, allowing reactions to occur faster and with less energy. The most common type of catalyst in living things are enzymes, which are proteins that catalyze biochemical reactions and help processes like digestion occur quicker without changing their own structure.



