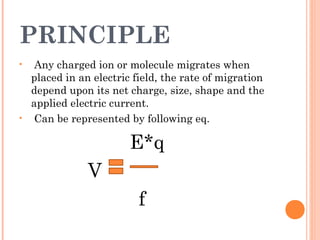
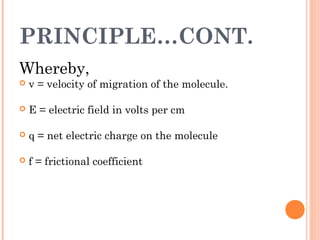
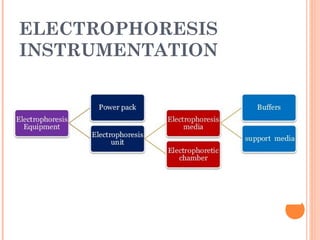
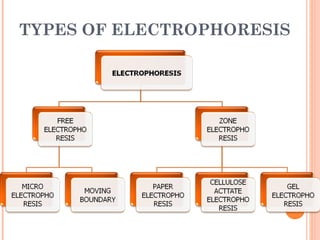
Polyacrylamide gel electrophoresis (PAGE) is a widely used technique for the separation of charged molecules in an electric field, influenced by their charge, size, and shape. The gel can be made from acrylamide and is known to separate smaller molecules, and can be visualized using various staining methods. SDS-PAGE is a modified form that denatures proteins to allow accurate separation based on their molecular weight.