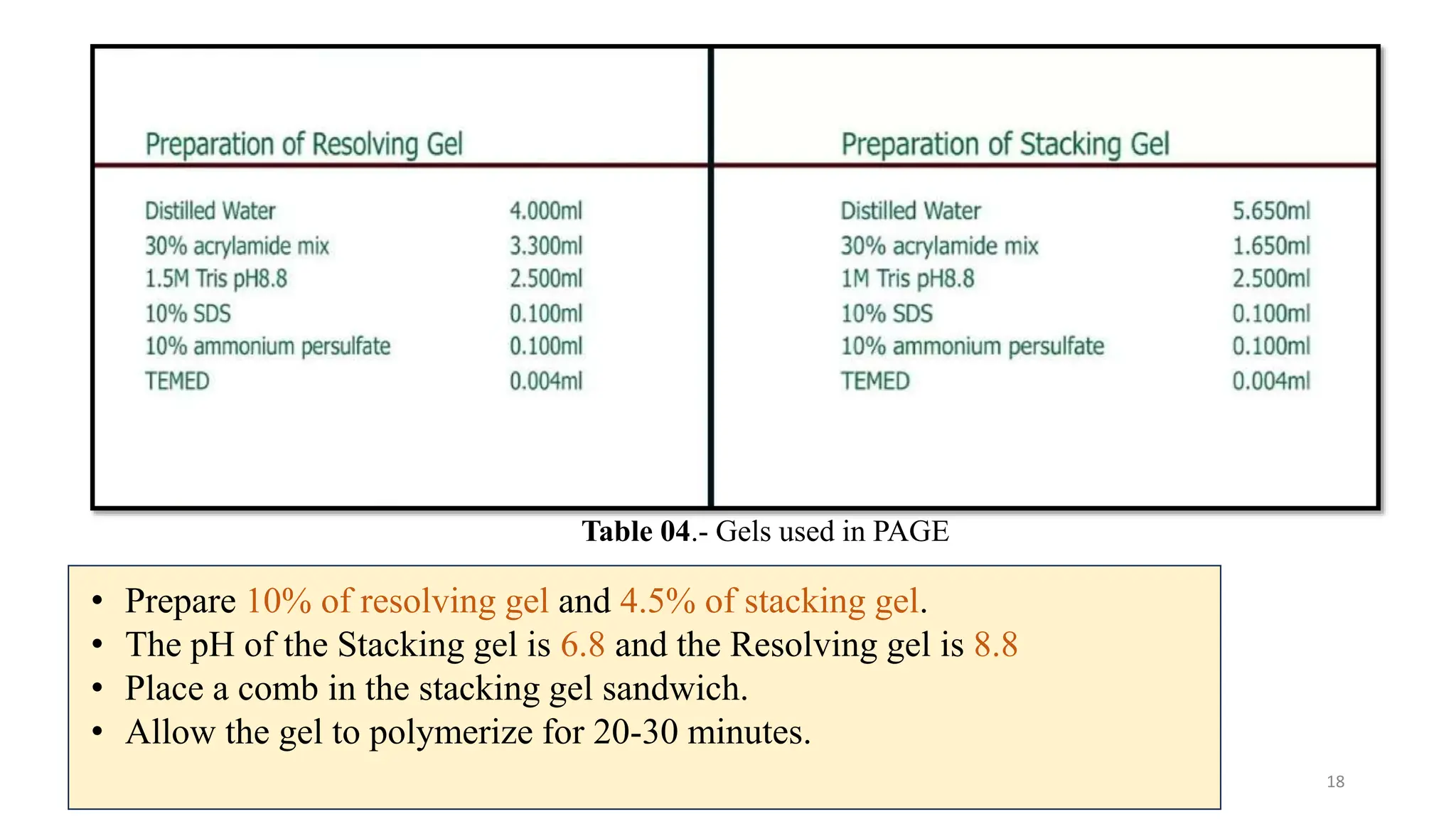
The document provides a detailed overview of gel electrophoresis, including its definition, principles, types, methodology, and applications. It discusses factors affecting electrophoresis and compares agarose and polyacrylamide gels, highlighting their advantages and disadvantages. The document also outlines the requirements for conducting gel electrophoresis and its applications in molecular biology, such as DNA analysis and protein separation.