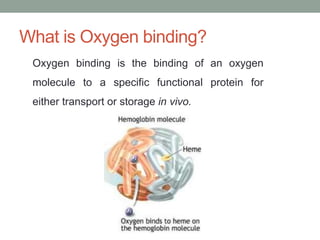
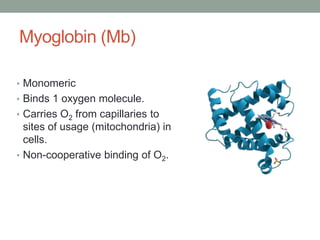
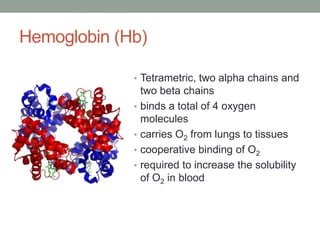
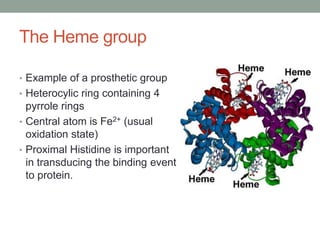
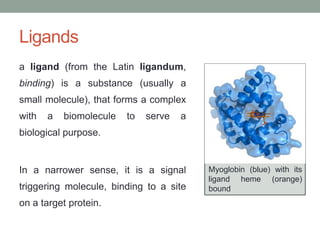
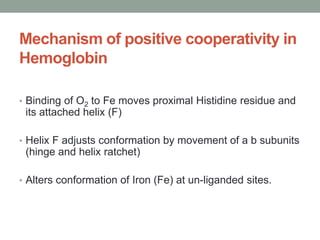
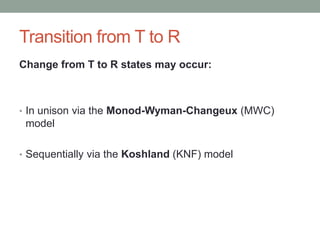
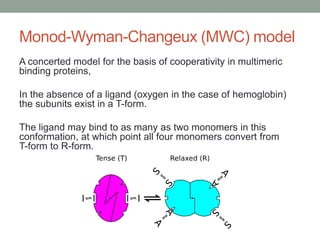
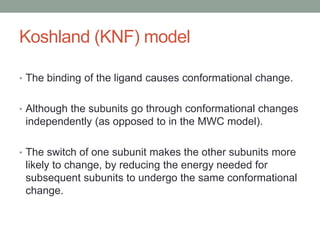
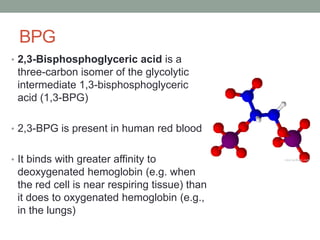
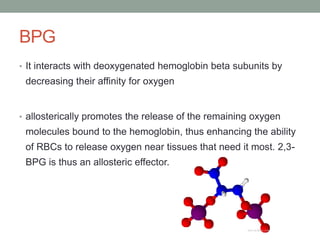
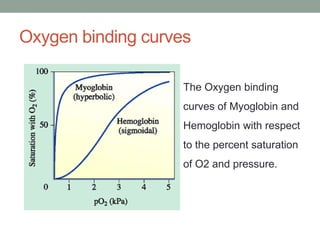
Oxygen binding proteins myoglobin and hemoglobin transport and store oxygen in the body. Myoglobin is a monomer that binds one oxygen molecule to carry oxygen to mitochondria in cells. Hemoglobin is a tetramer made of two alpha and two beta chains that binds four oxygen molecules cooperatively to carry oxygen from lungs to tissues. The heme group contains iron that binds oxygen. Hemoglobin exhibits positive cooperativity where binding of oxygen to one subunit increases the affinity of the other subunits. The Monod-Wyman-Changeux and Koshland models describe the transition between tense and relaxed hemoglobin states during cooperativity. 2,3-bisphosphoglycerate binds preferentially to deoxygenated hemoglobin and shifts