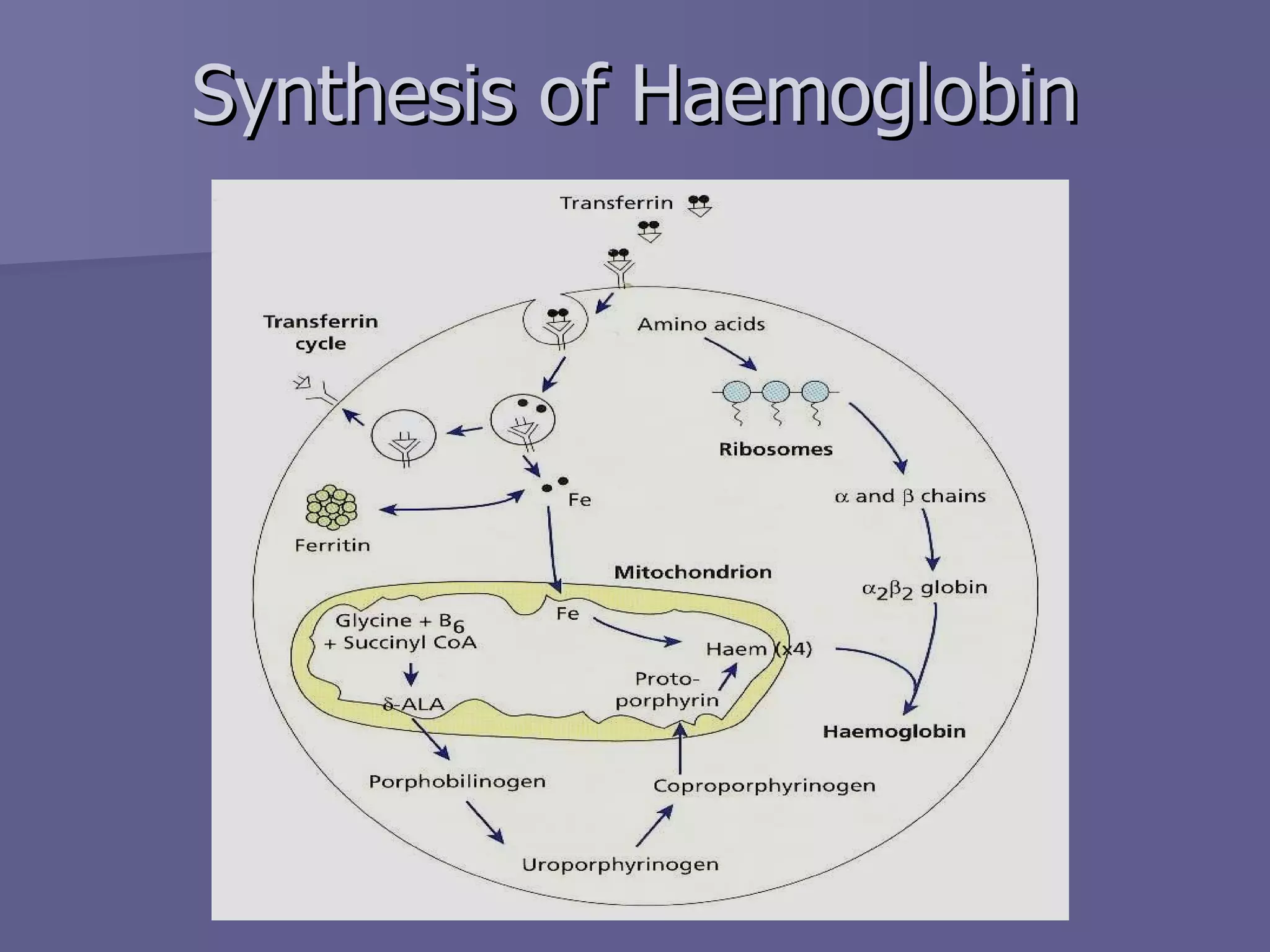
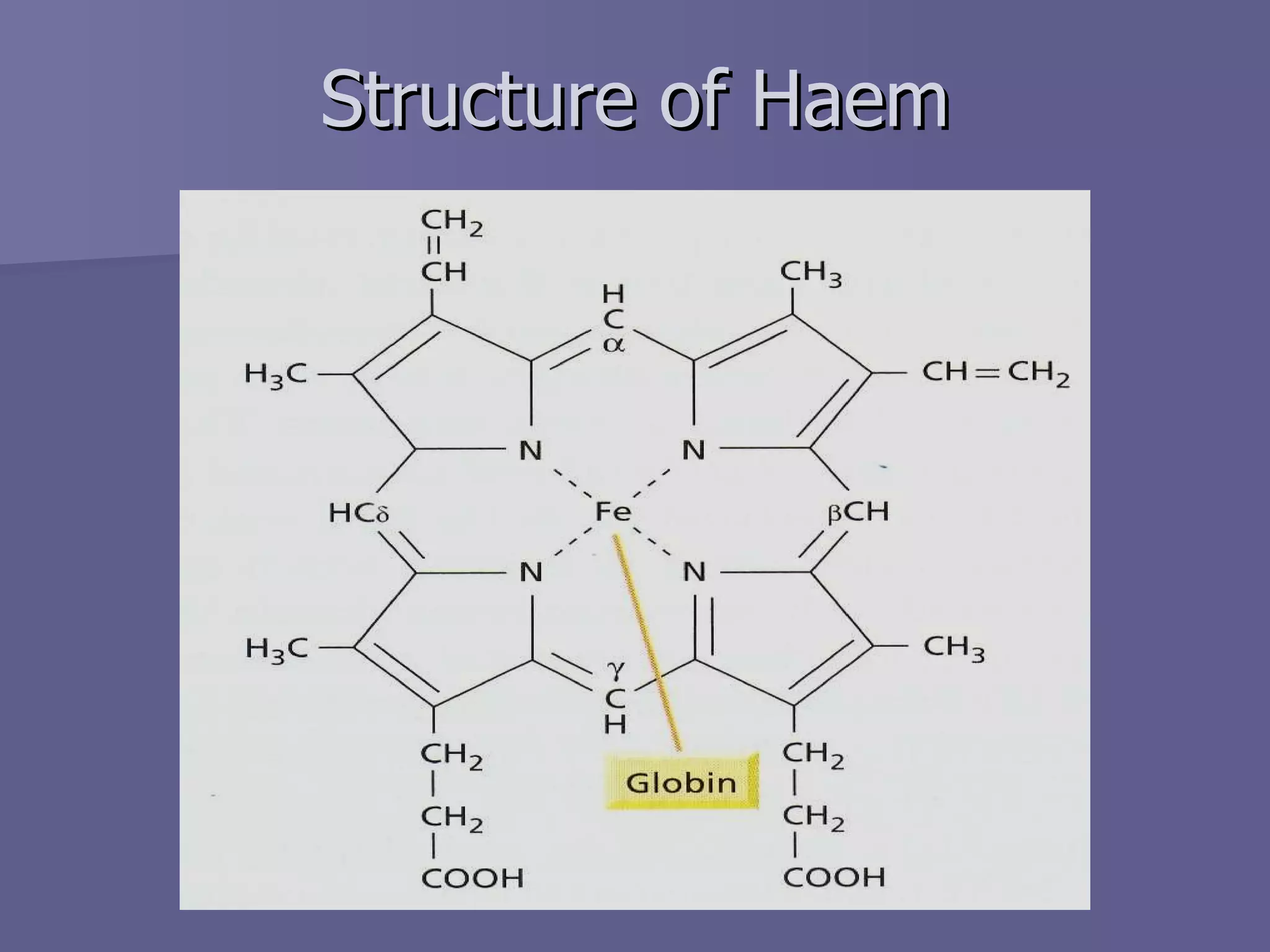
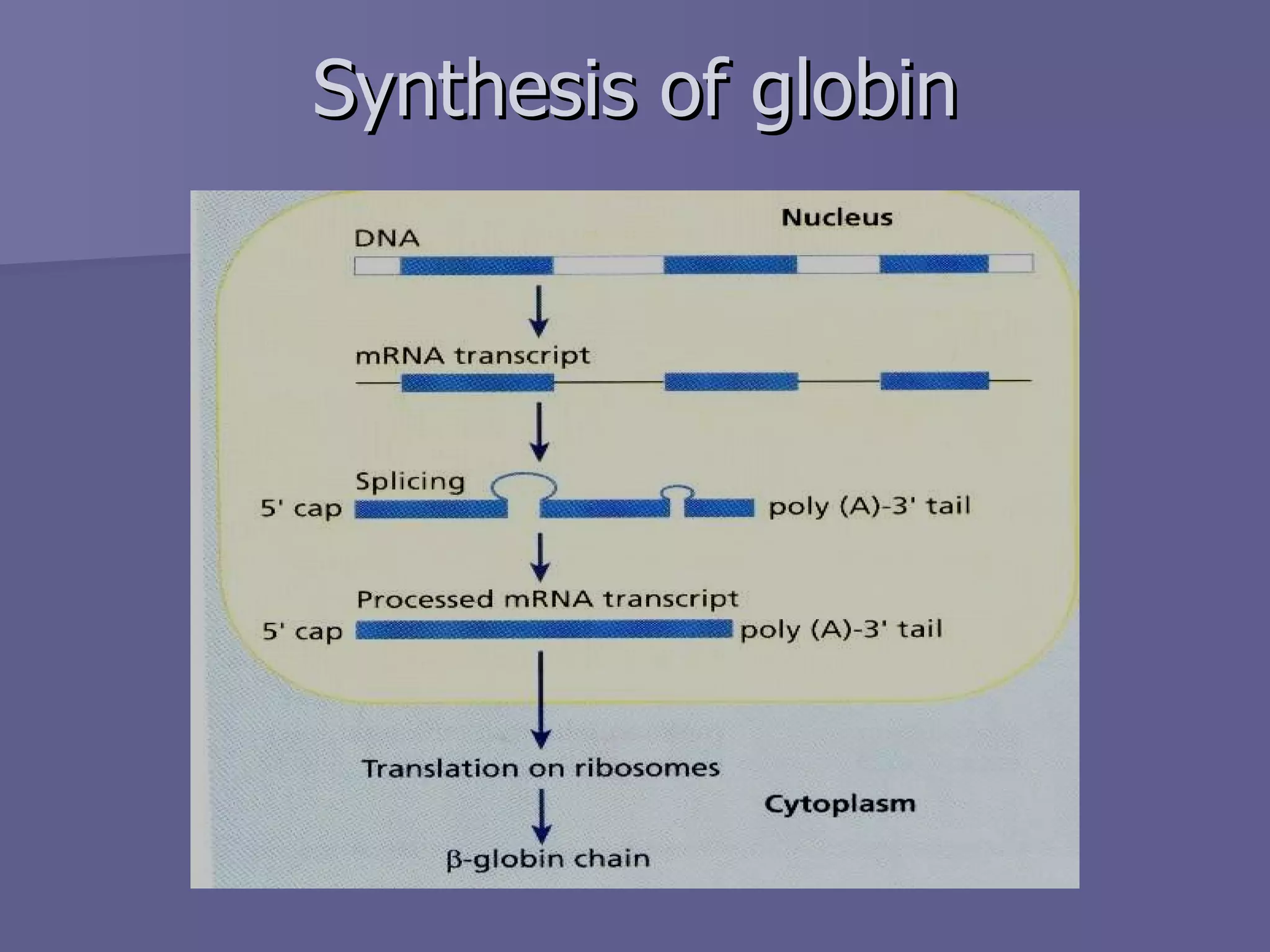
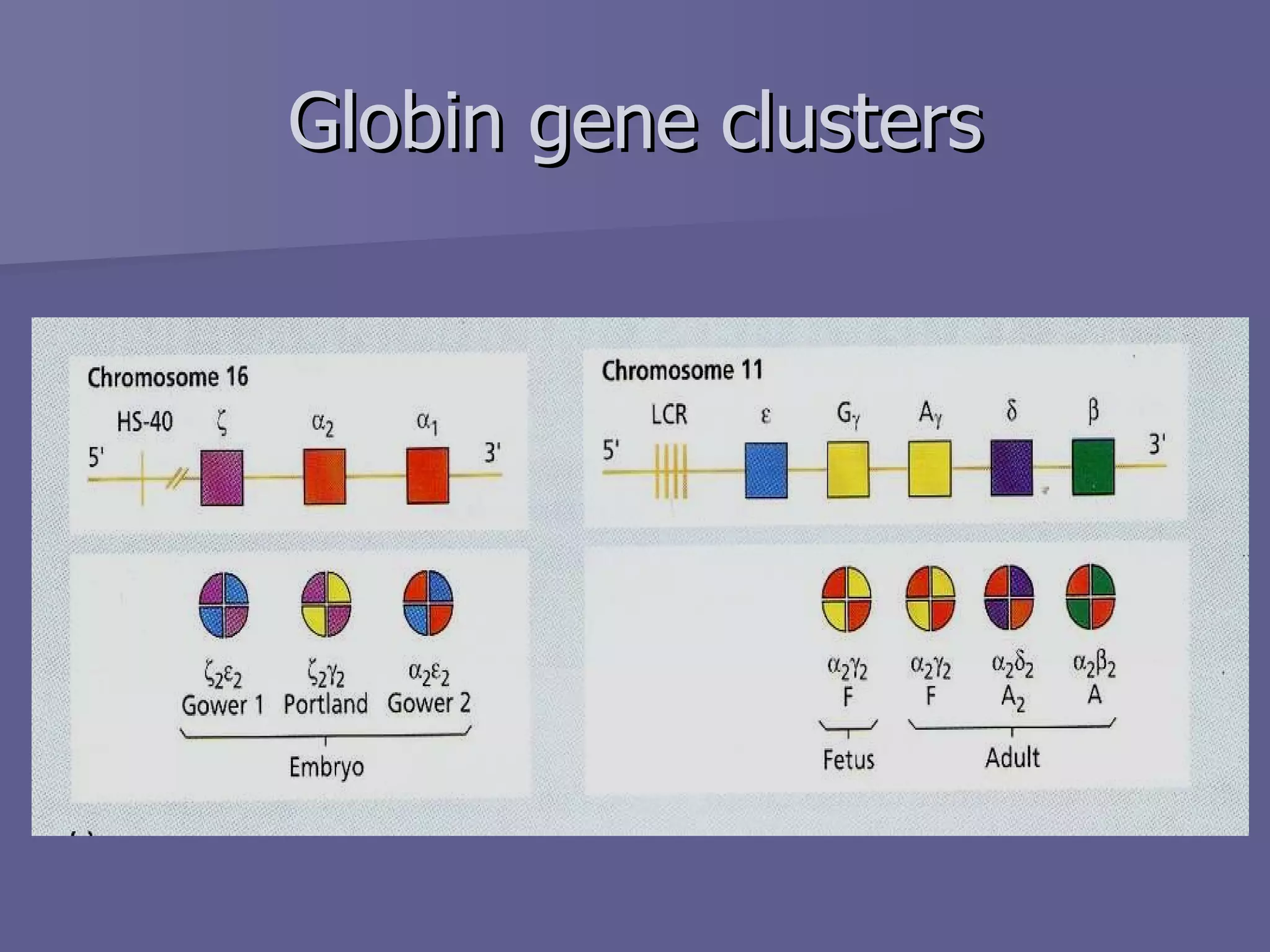
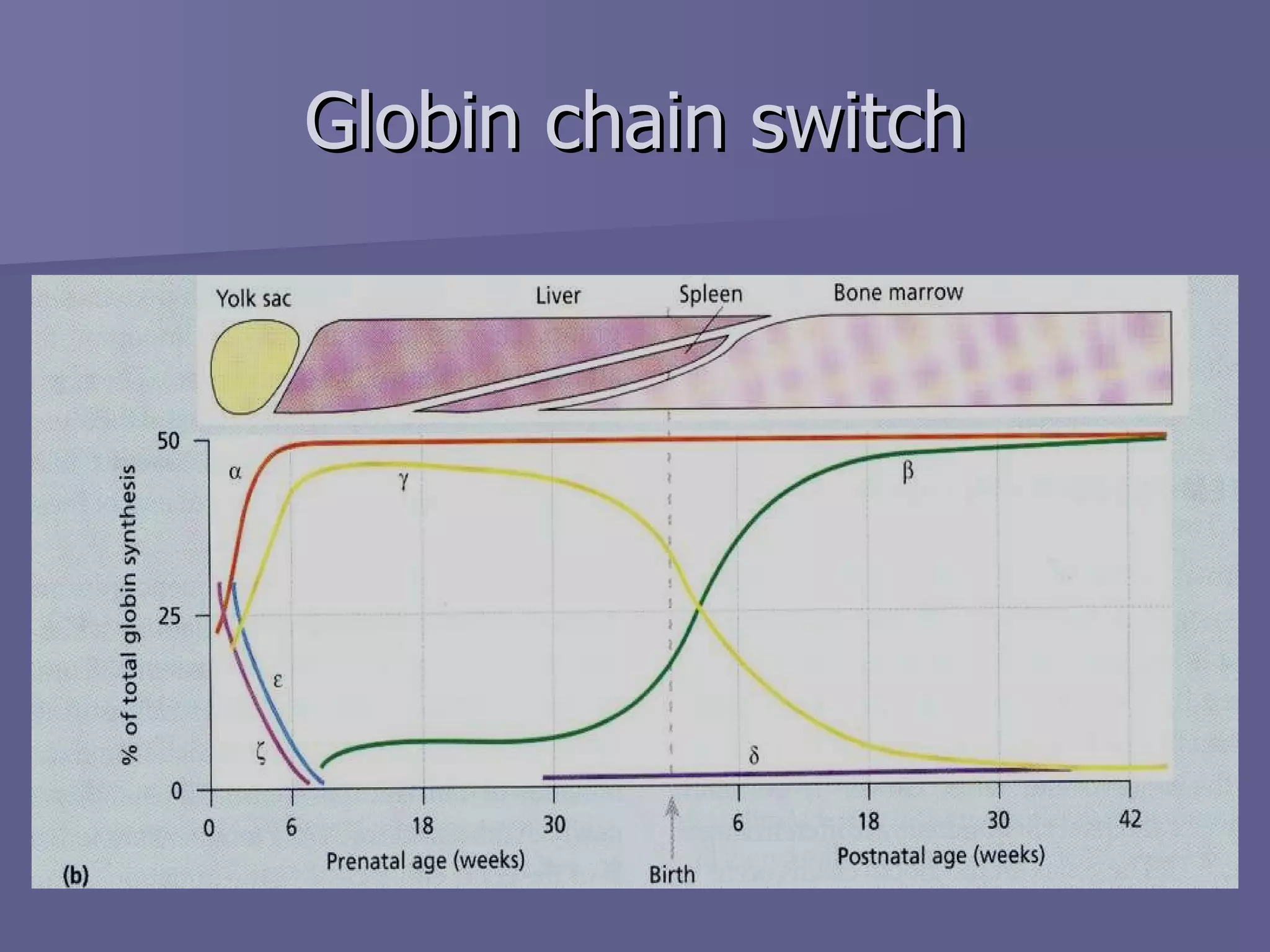
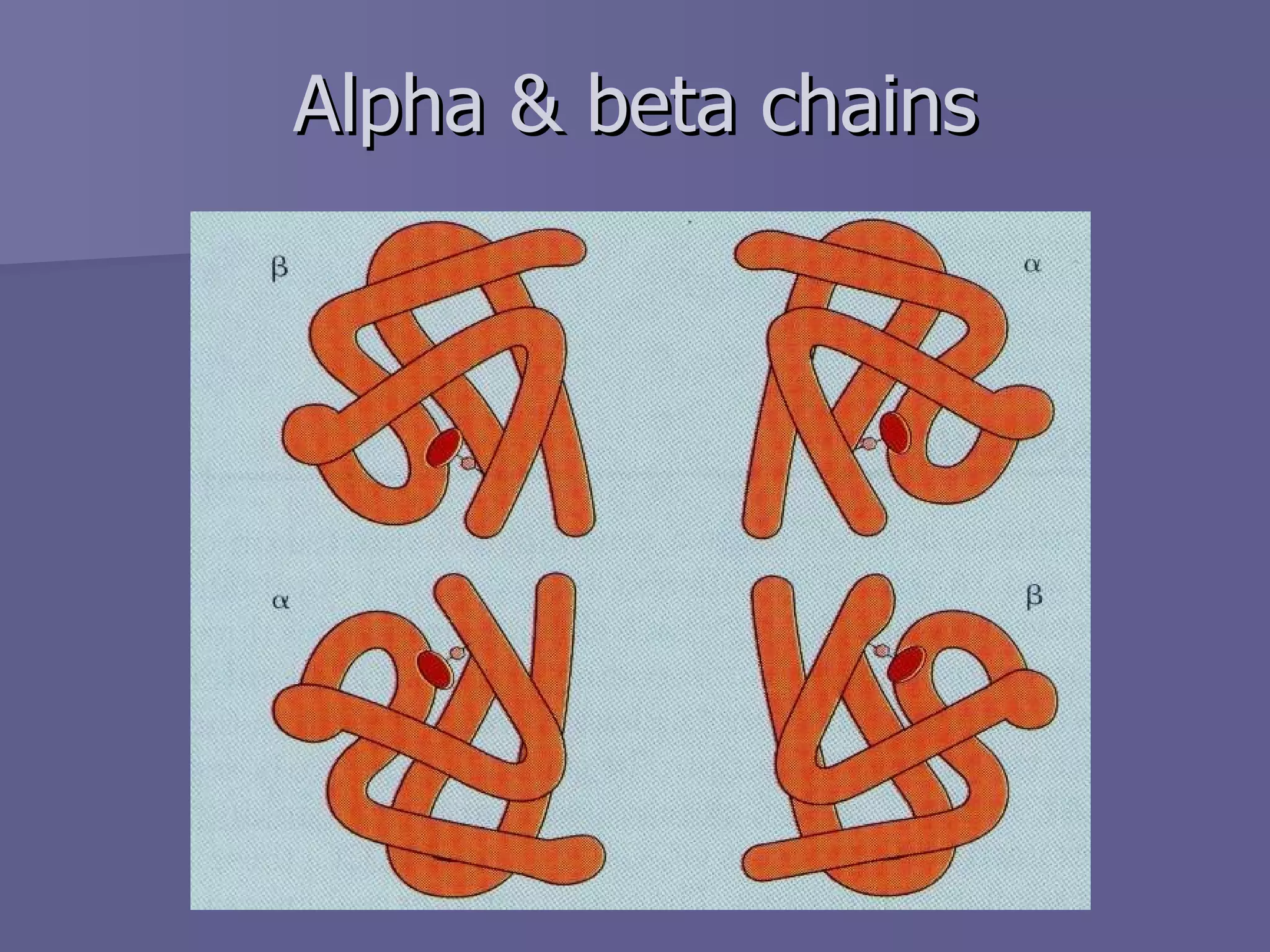
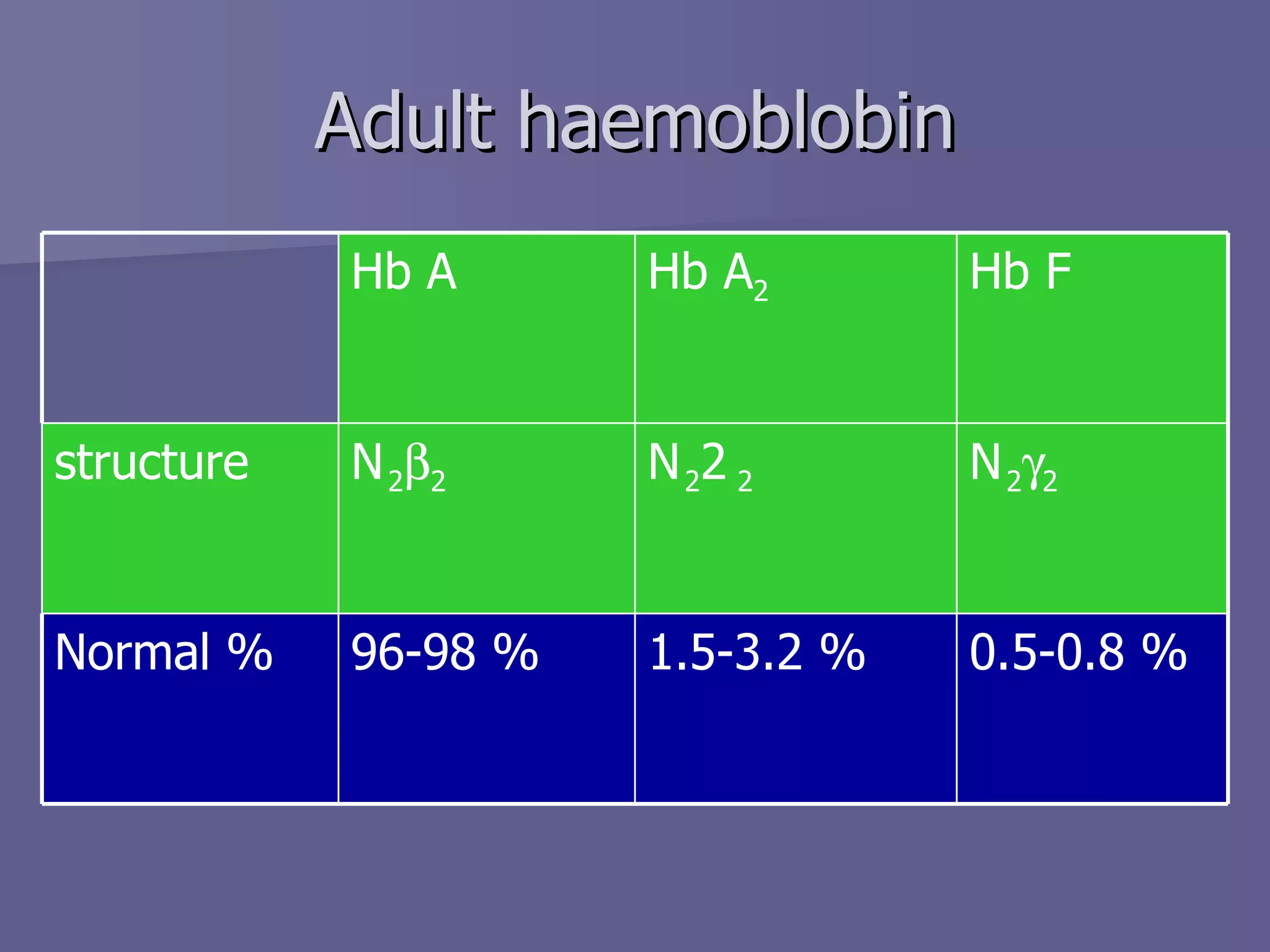
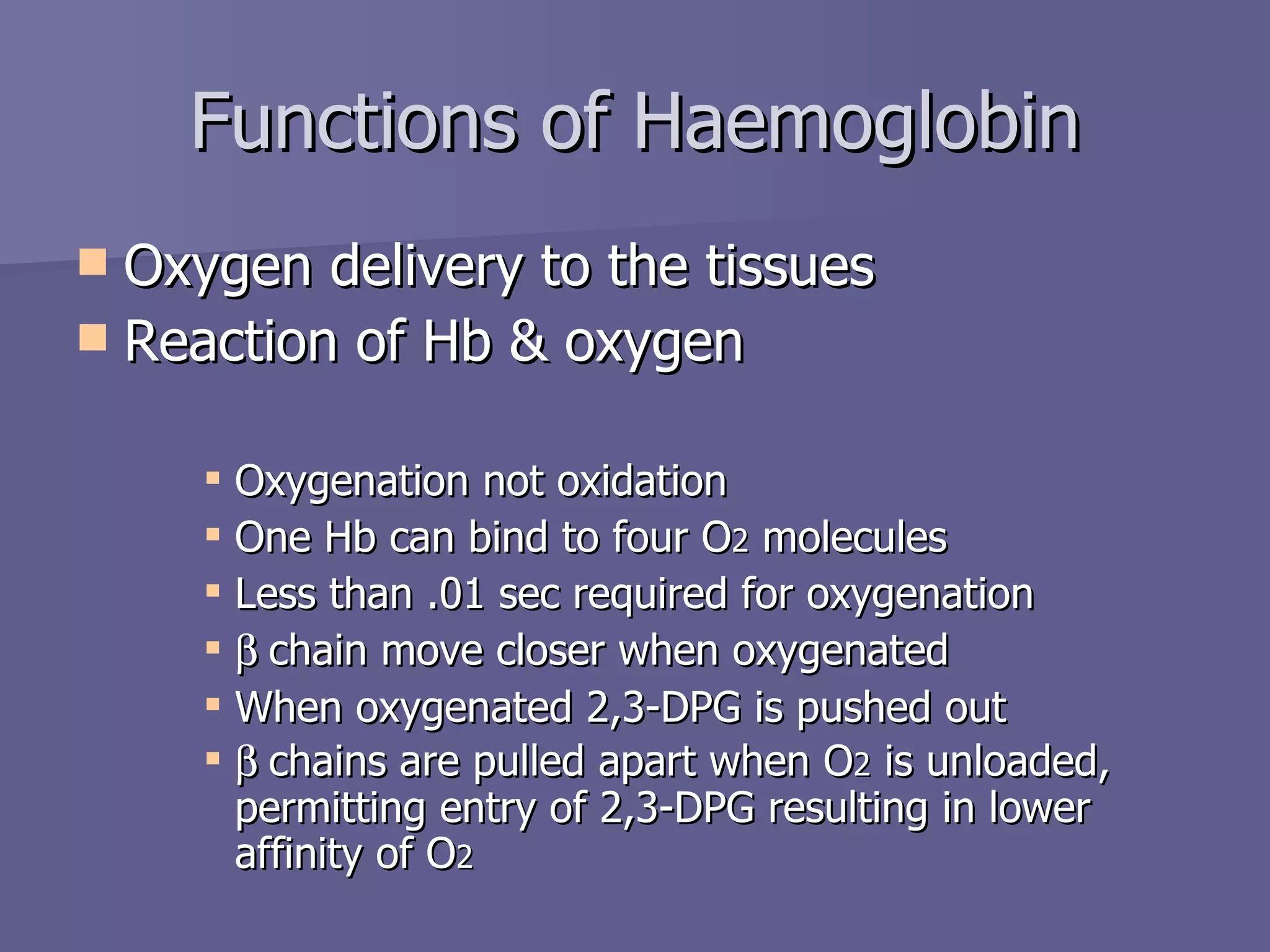
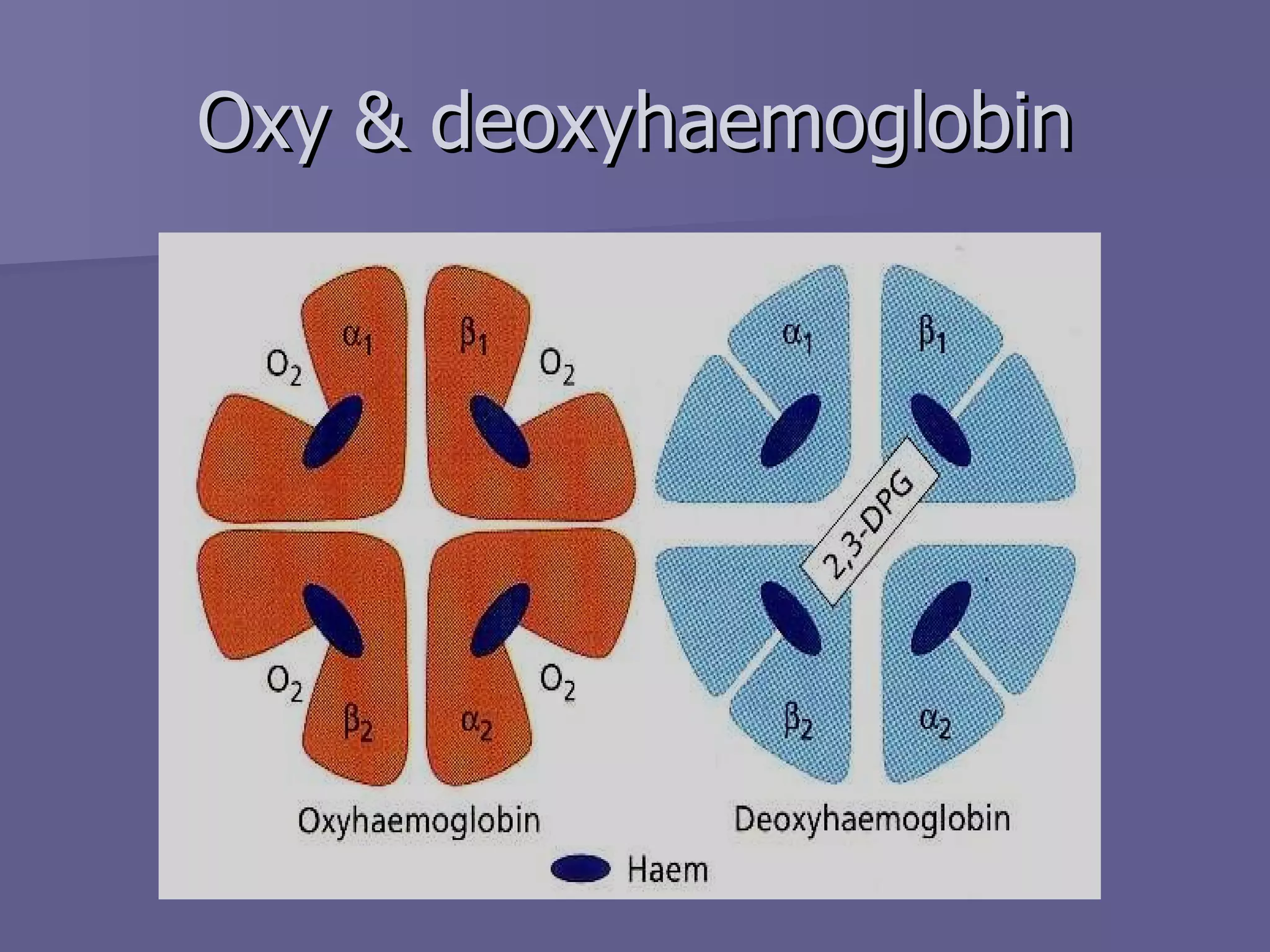
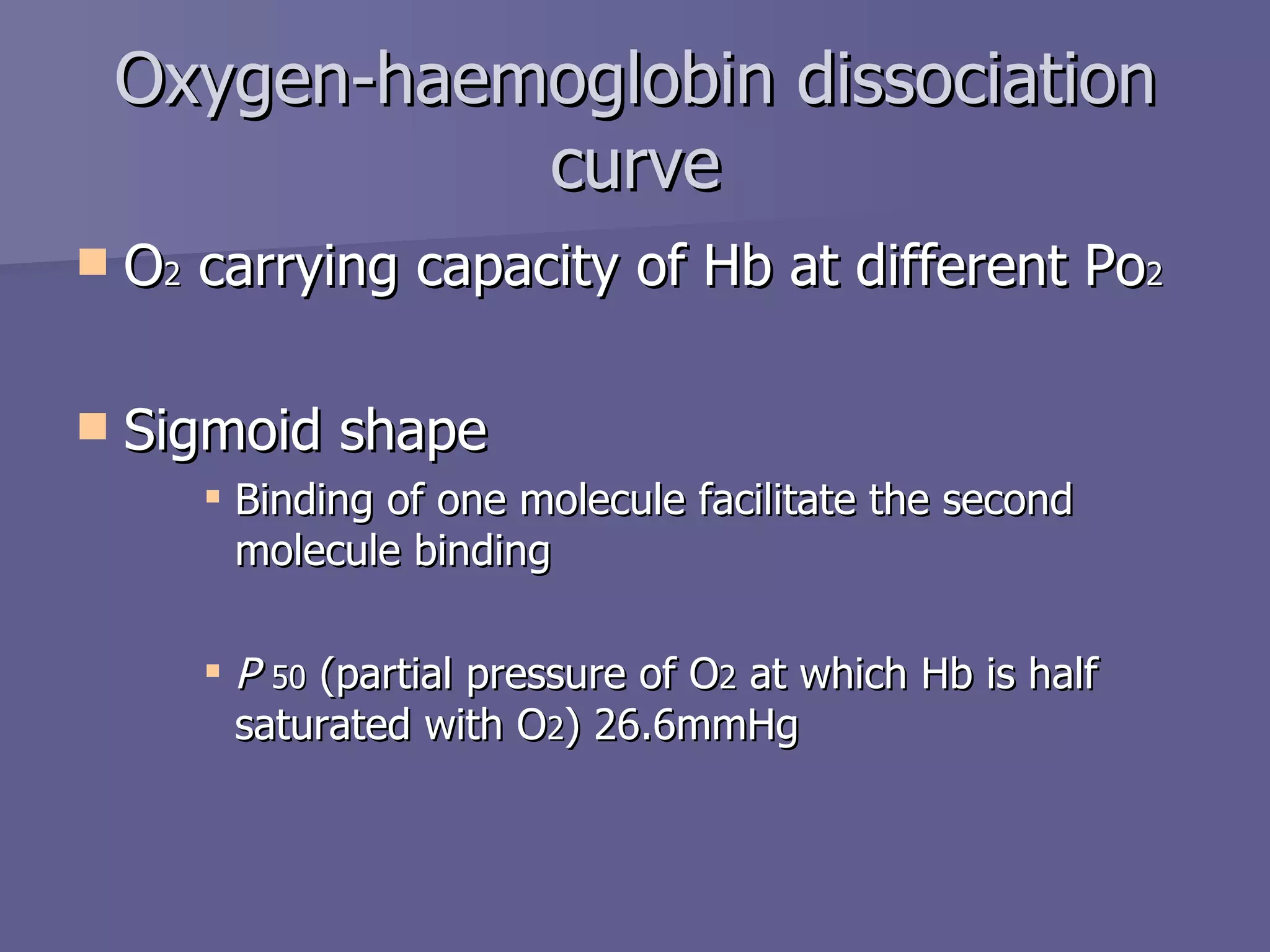
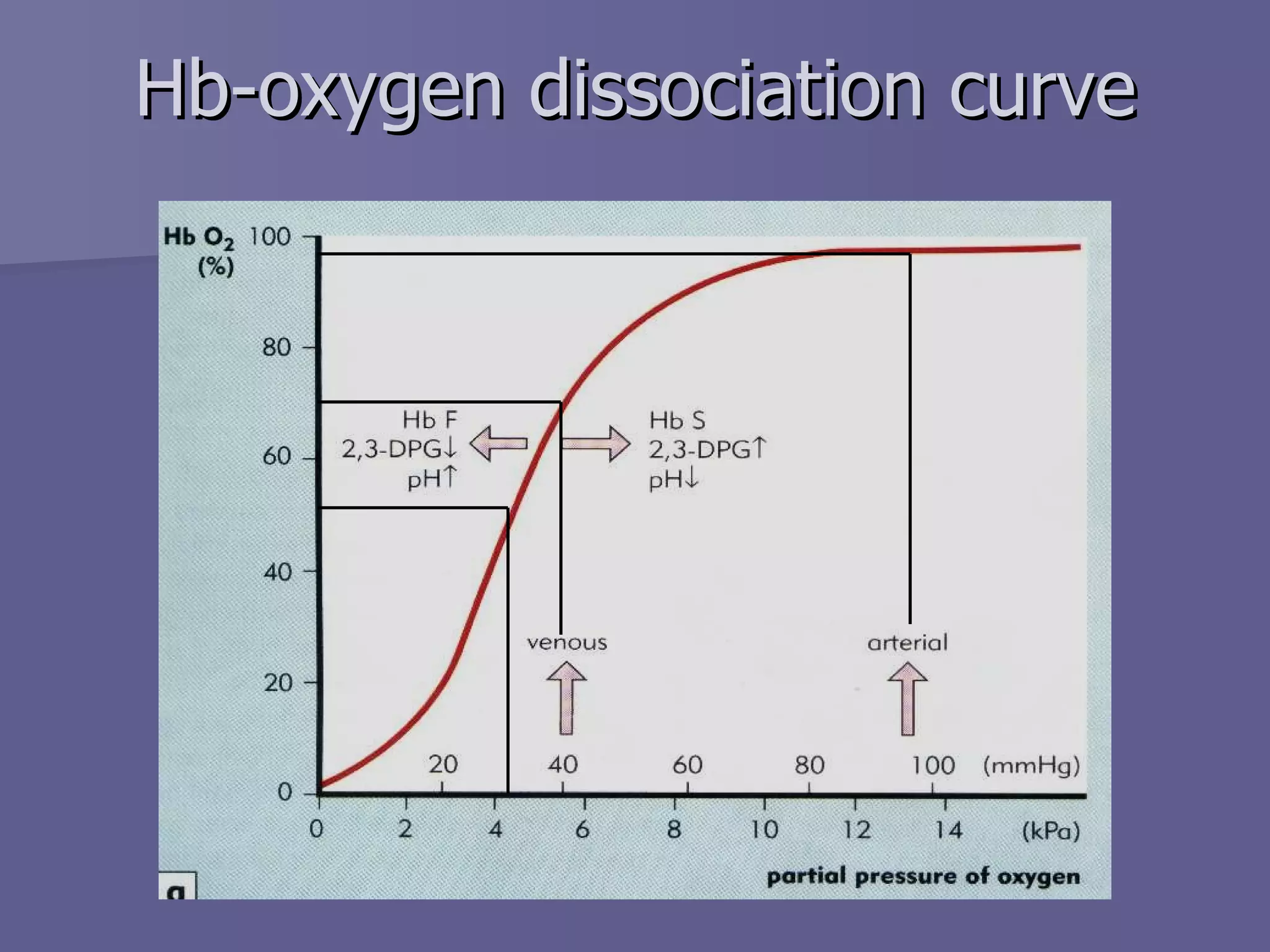
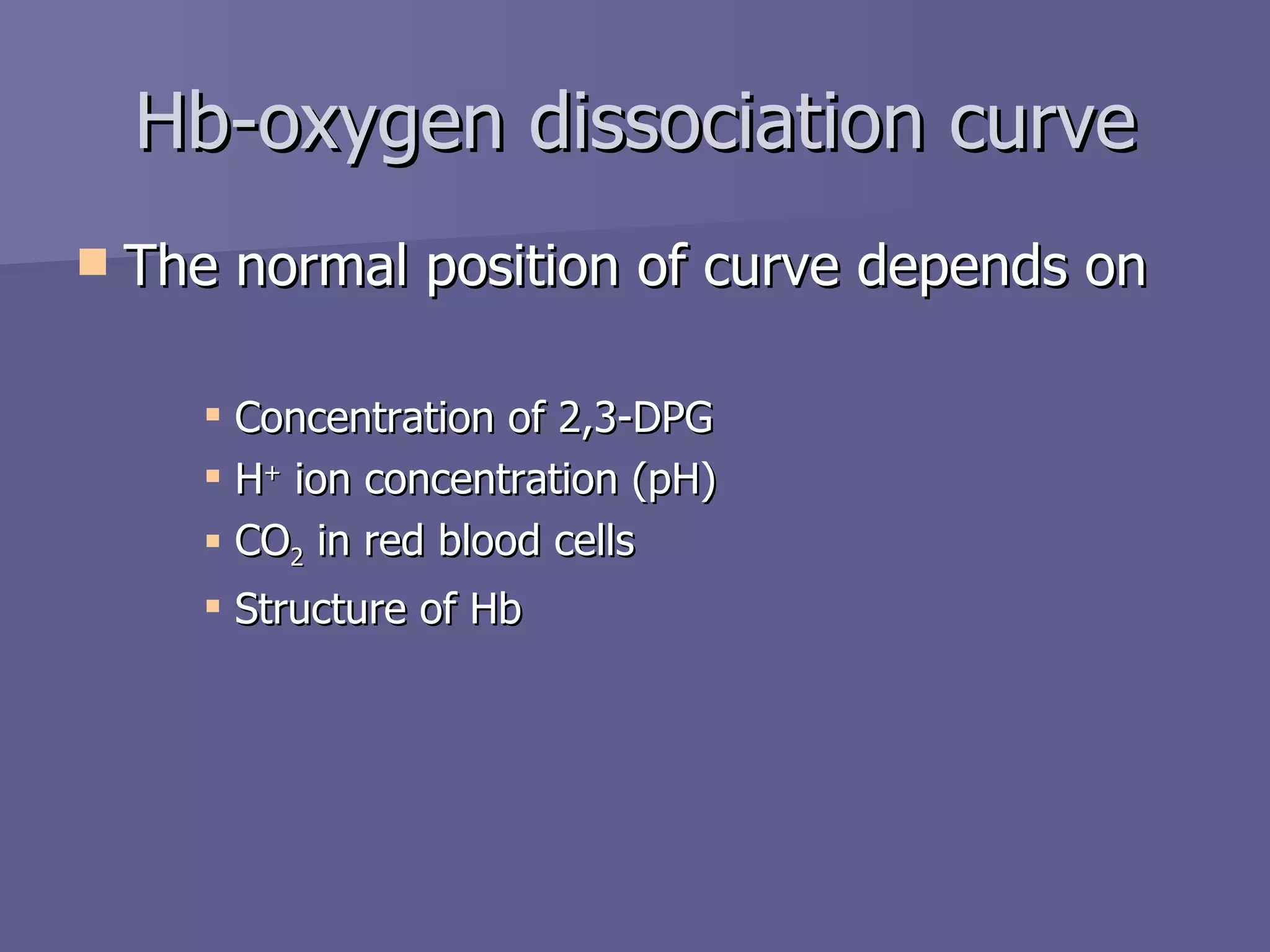
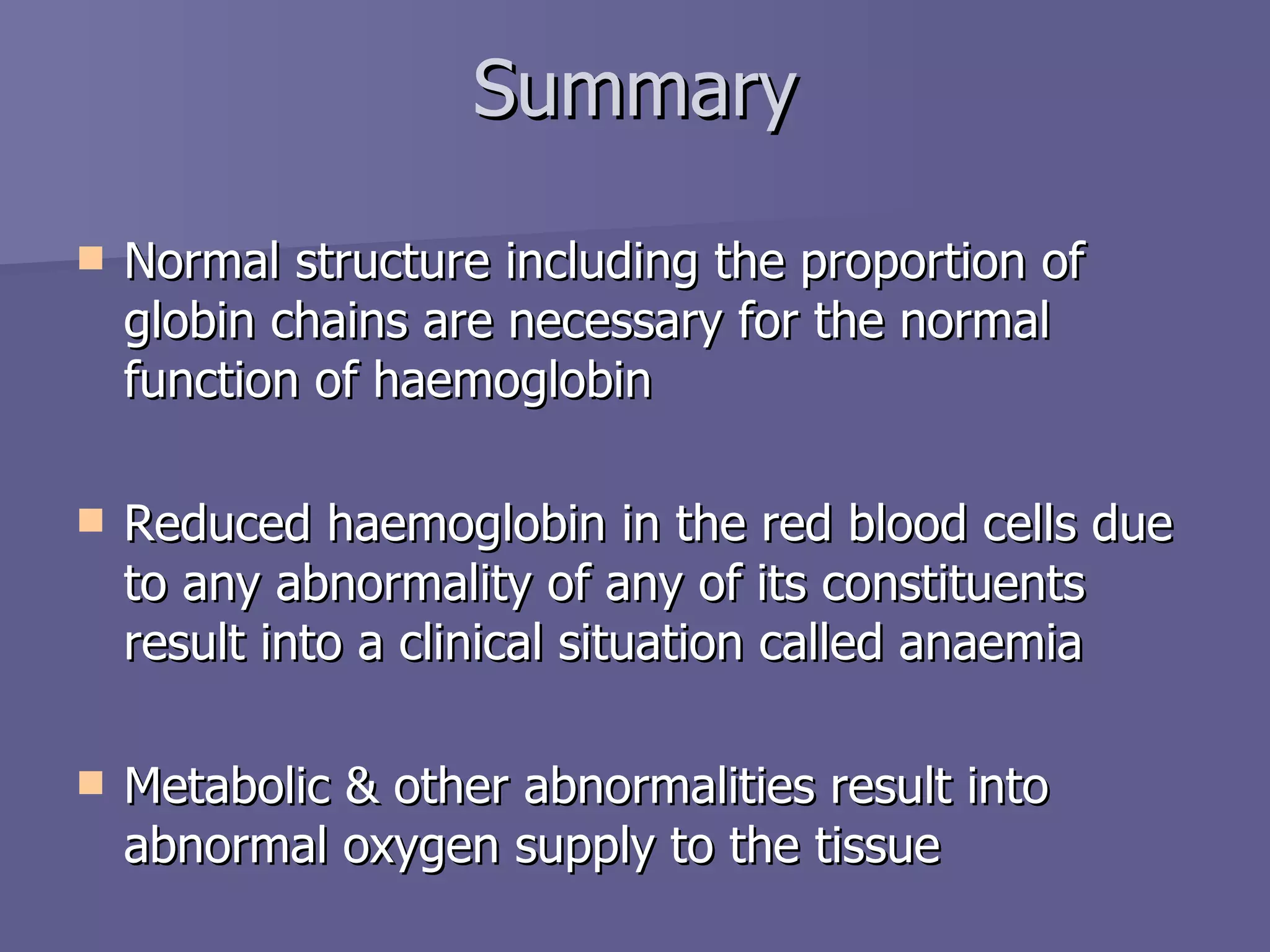
The document summarizes the structure and function of hemoglobin. Hemoglobin is a protein in red blood cells that carries oxygen from the lungs to tissues and carbon dioxide from tissues back to the lungs. It is composed of heme and globin proteins. Heme contains iron and is produced in mitochondria, while globin chains are produced by ribosomes and combine with heme to form hemoglobin. The main types of hemoglobin in humans are fetal hemoglobin during development and adult hemoglobin after birth. Hemoglobin transports oxygen via an oxygen-binding reaction that allows it to efficiently deliver oxygen to tissues and receive carbon dioxide.