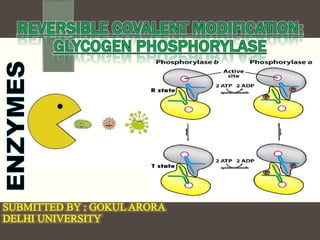
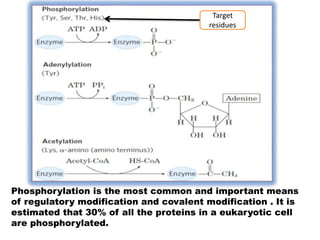
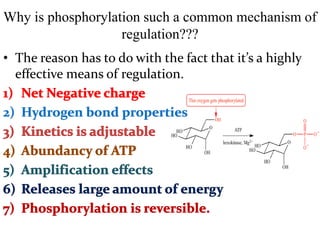
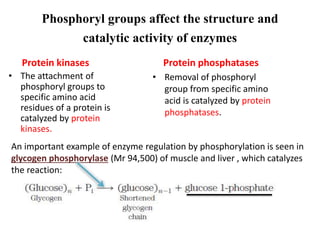
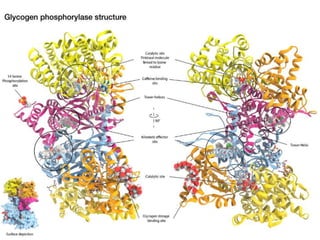
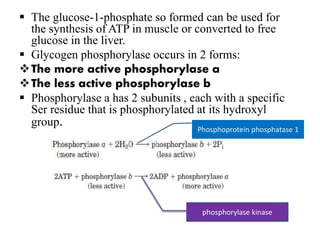
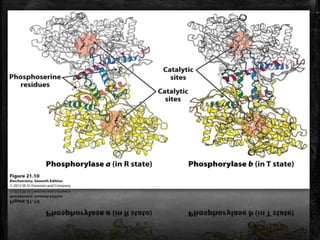
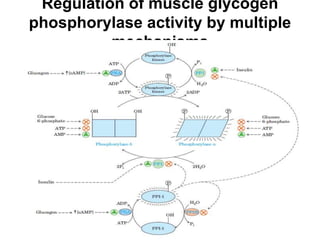
This document discusses the regulation of enzymes through covalent modification, with a focus on phosphorylation. It states that phosphorylation is the most common form of covalent modification, occurring in 30% of eukaryotic proteins. Phosphorylation involves the addition of phosphoryl groups to amino acids by protein kinases and removal by protein phosphatases. This effectively regulates enzyme activity and structure. A key example is regulation of glycogen phosphorylase in muscle and liver through interconversion between active phosphorylated form A and less active form B by phosphorylation and dephosphorylation. Glycogen phosphorylase breakdown is regulated by controlling the ratio of these two forms.