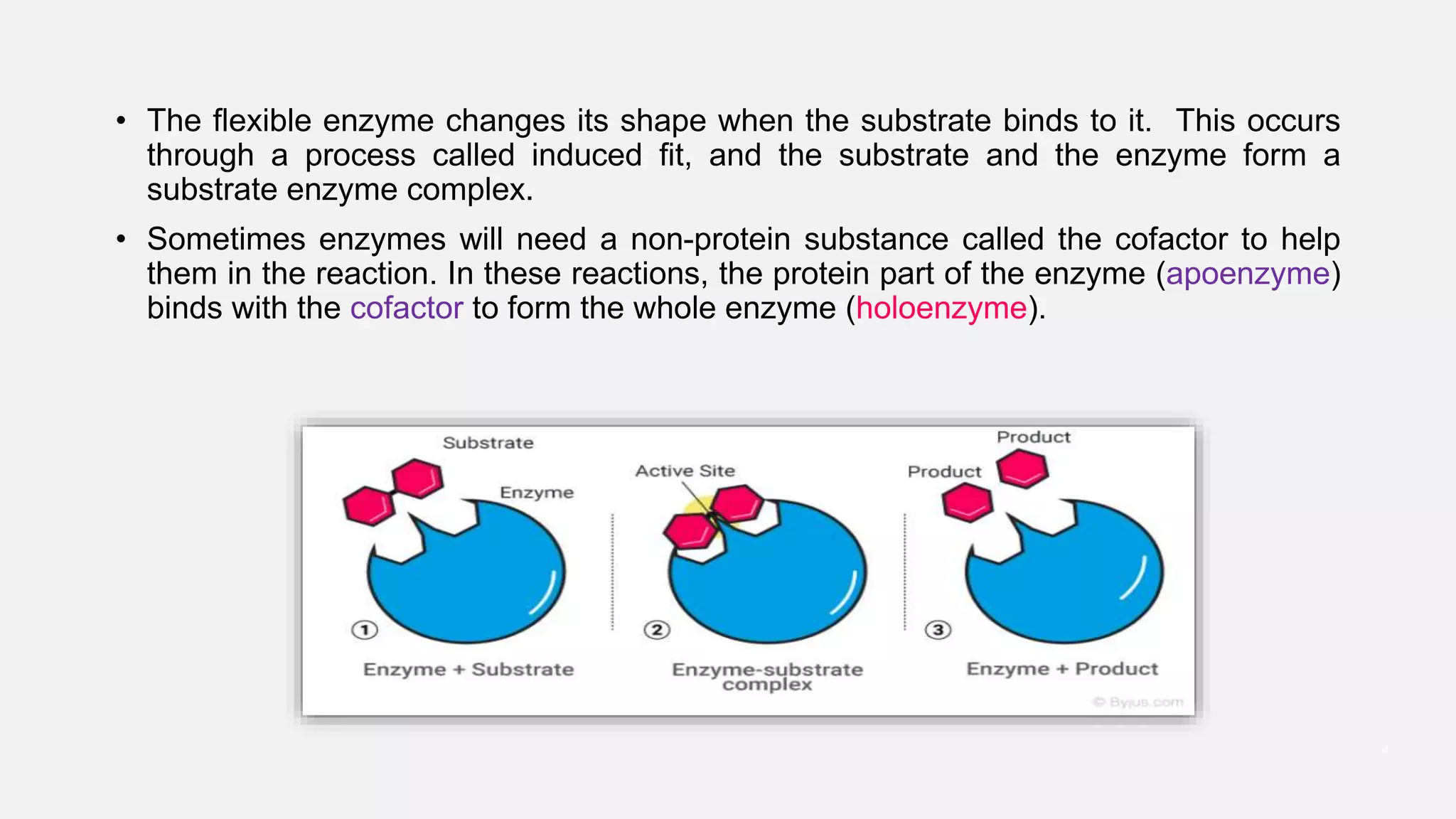
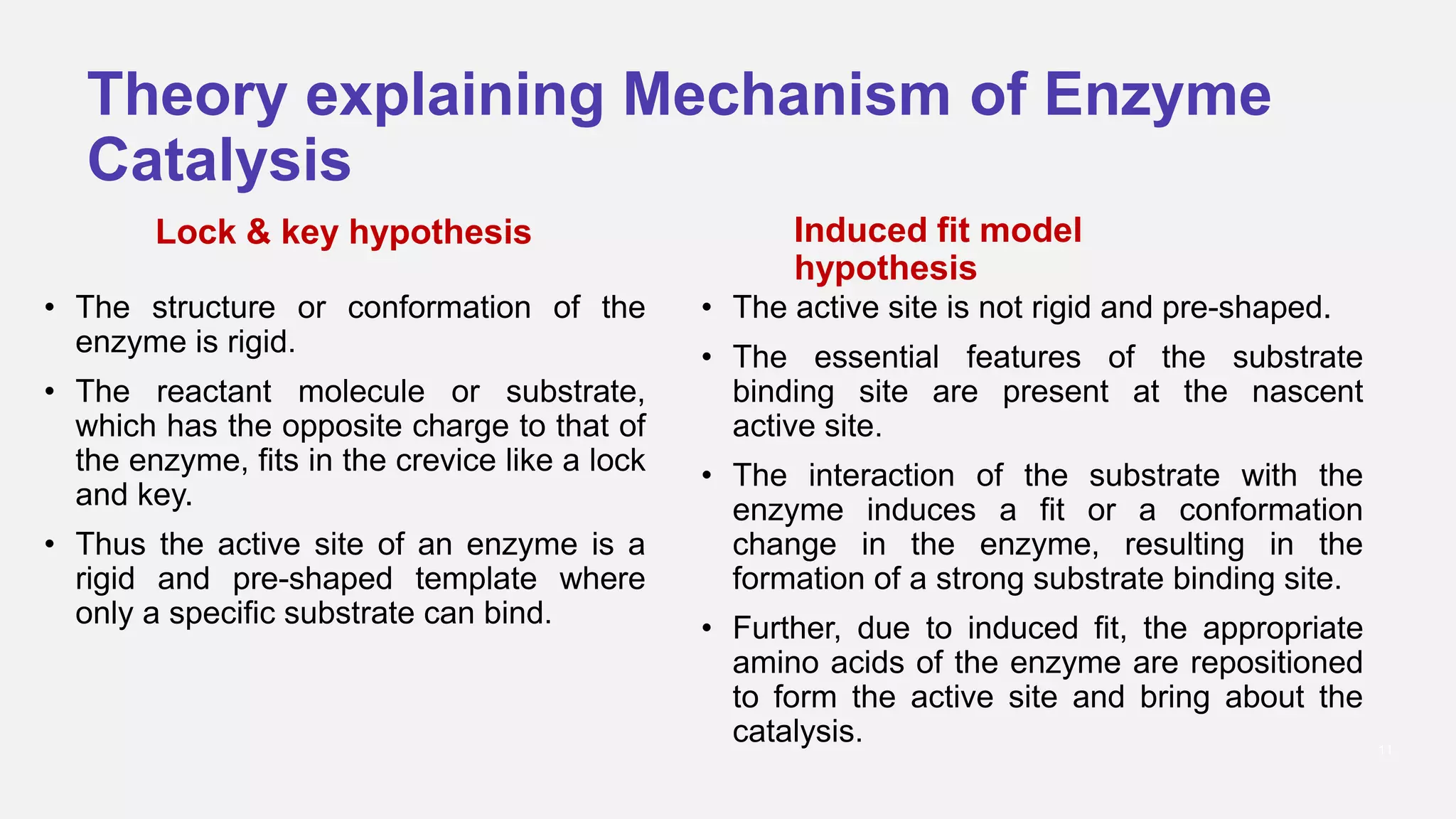
Enzyme catalysis significantly accelerates biochemical reactions by lowering the activation energy needed for substrates to convert into products, primarily through the formation of an enzyme-substrate complex at the active site. The process is highly specific and efficient, with enzymes functioning optimally at specific temperatures and pH levels, and often requiring additional cofactors for full activity. Various mechanisms such as induced fit, acid-base catalysis, and proximity effects all contribute to the overall function and effectiveness of enzymes in biological systems.