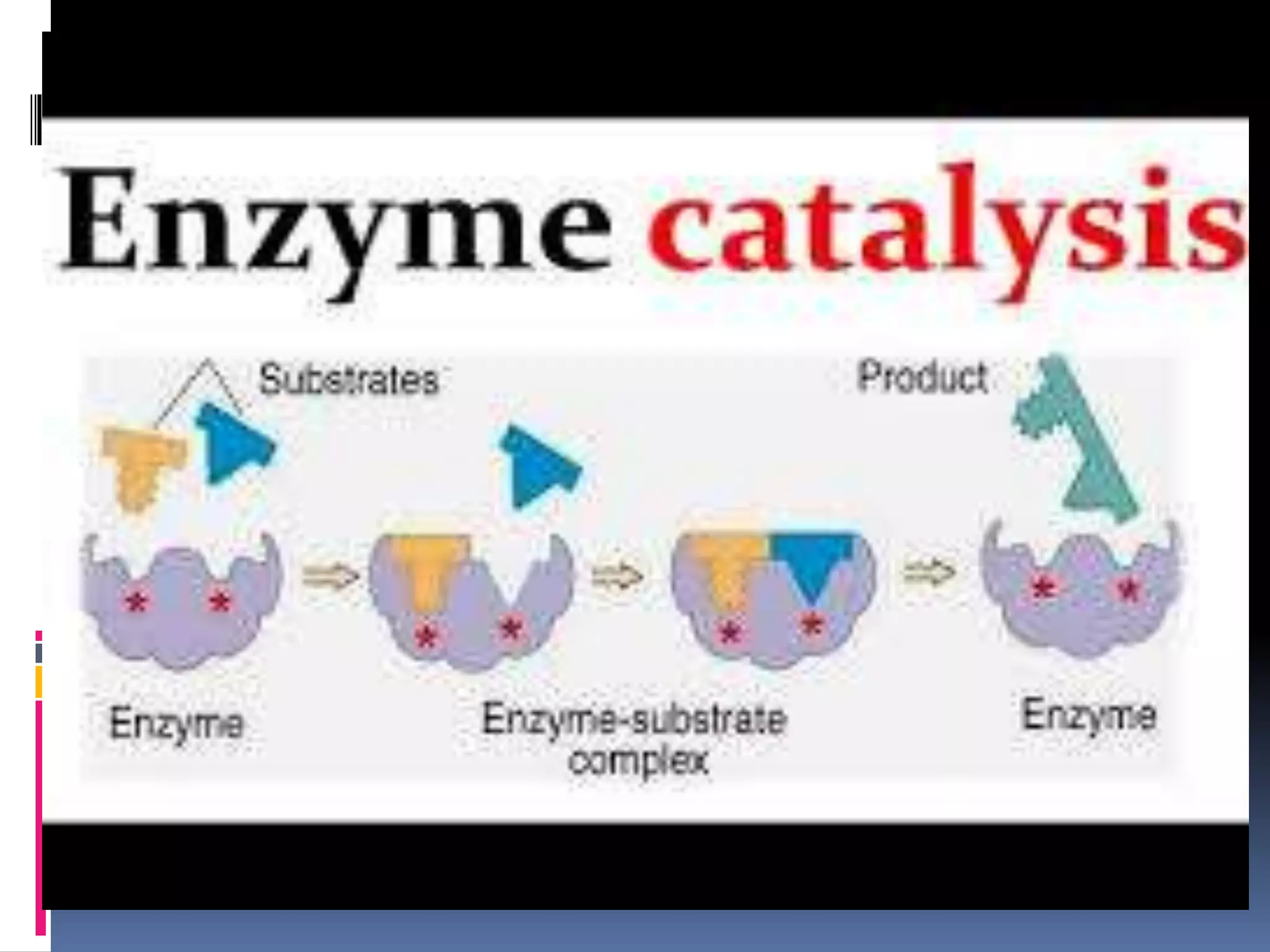
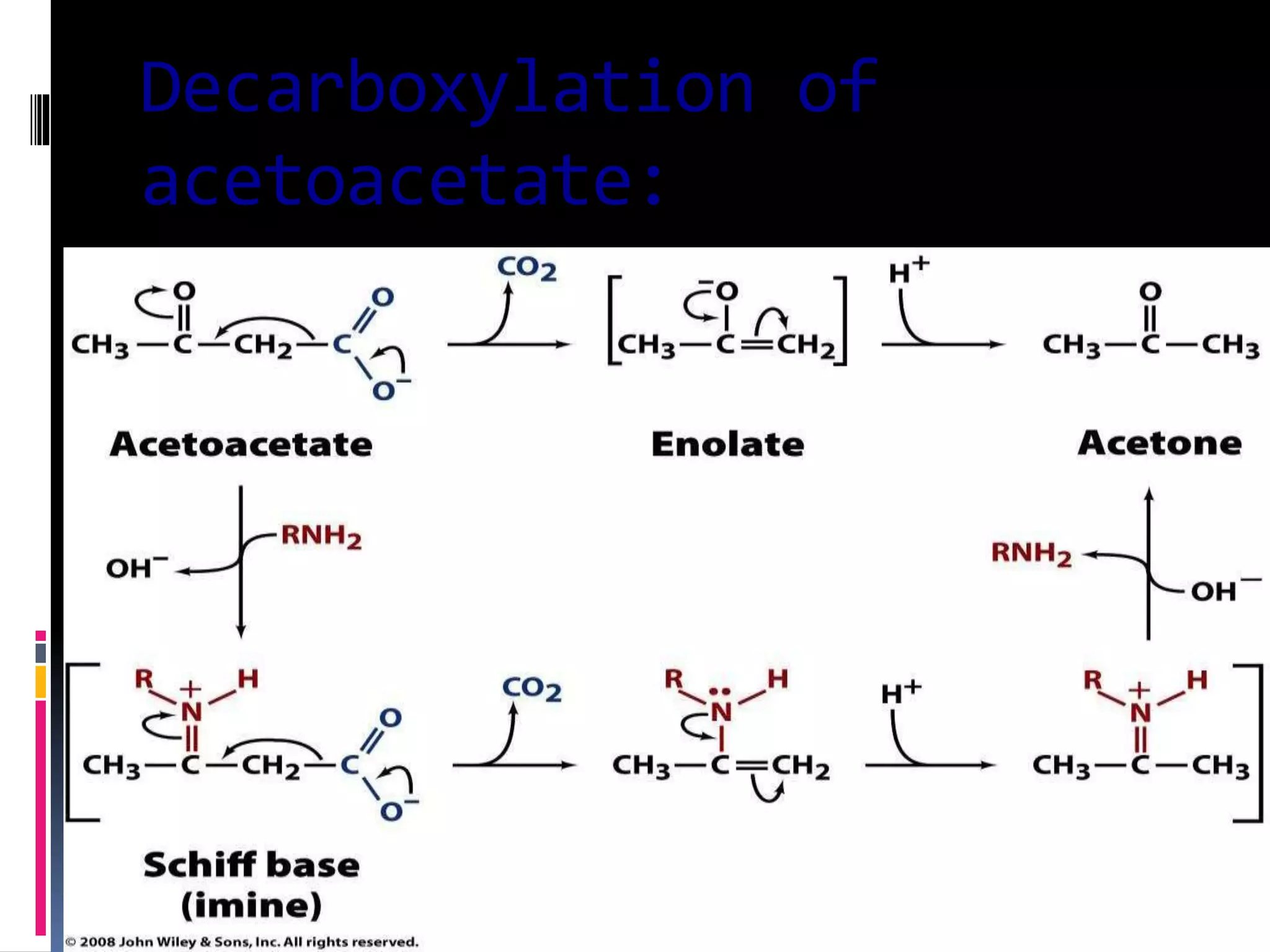
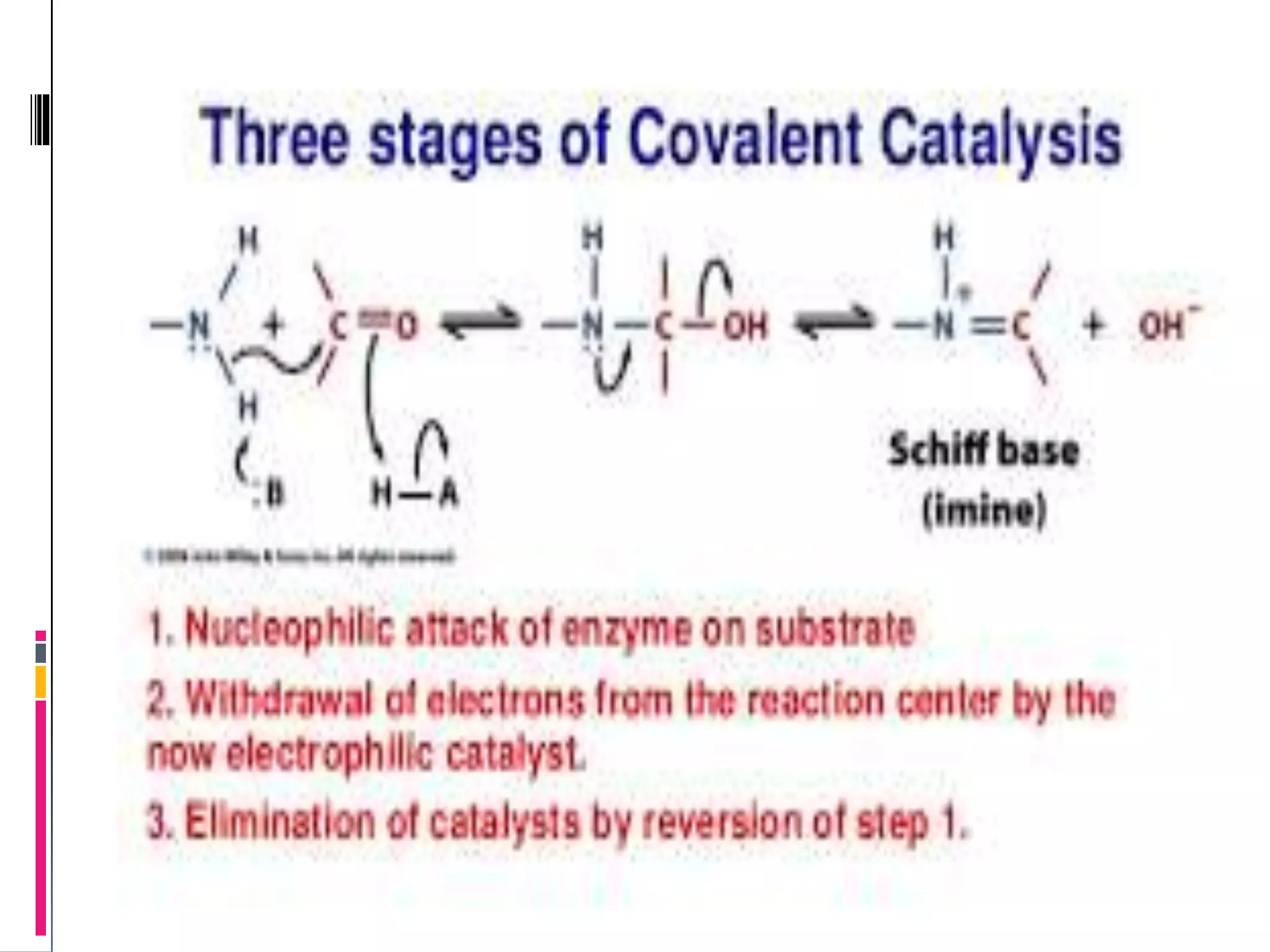
This document discusses two types of enzyme catalysis: covalent and electrostatic. Covalent catalysis involves the transient formation of a covalent bond between an enzyme's nucleophilic group (such as an amino acid side chain) and an electrophilic group on the substrate. This is exemplified by the decarboxylation of acetoacetate through Schiff base formation with a lysine residue. Electrostatic catalysis enhances reaction rates through the arrangement of charged groups within the enzyme's active site to stabilize transition states. The local low dielectric environment of the active site strengthens electrostatic interactions.