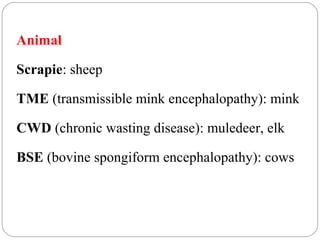
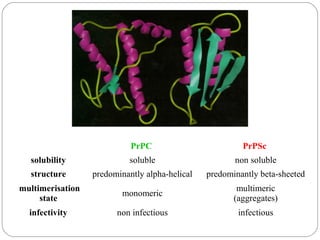
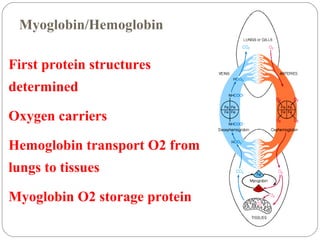
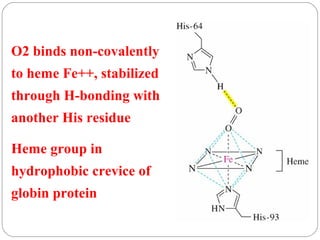
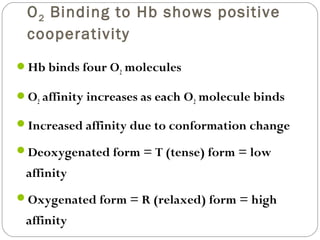
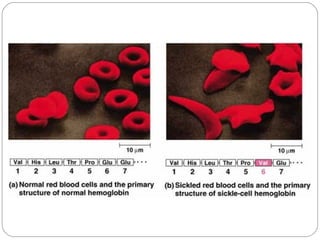
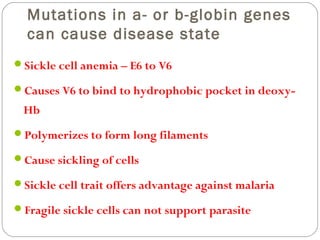
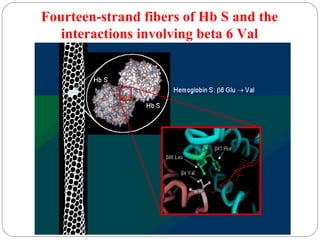
Prion diseases can affect various animal species, including sheep (scrapie), mink (TME), deer and elk (CWD), and cows (BSE). In humans, prion diseases include CJD, FFI, and kuru. The pathogenic prion protein (PrPSc) differs from the normal prion protein (PrPC) in having a higher beta-sheet content, being insoluble and able to aggregate, and being infectious. PrPSc converts PrPC into more PrPSc, propagating the disease. Hemoglobin and myoglobin are oxygen carrier proteins that contain heme groups and have similar alpha-helical structures, but hemoglobin is a heterotetramer that binds oxygen cooperatively