Hemoglobin and myoglobin are oxygen-carrying proteins in the blood. Hemoglobin is a tetrameric protein composed of four polypeptide chains that carry oxygen from the lungs to tissues and carbon dioxide from tissues back to the lungs. Myoglobin is a single-chain protein that stores oxygen in muscle tissues. Both proteins use heme groups containing iron to reversibly bind oxygen. The binding of oxygen is influenced by factors like pH, carbon dioxide levels, and 2,3-bisphosphoglycerate to facilitate oxygen delivery to tissues and carbon dioxide removal from tissues.



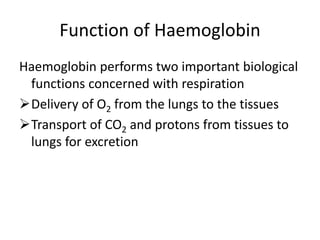
![(R)
relaxed state
(T)
tense state
The Transportation of Blood Oxygen
Hemoglobin
Lung
O2
Myoglobin
Muscle
Vein
Artery
When environmental [O2]
increases, Hb binds oxygen
efficiently
When environmental [O2]
decreases, Hb releases
oxygen to Mb
Any one subunit receives an oxygen molecule will increase the
oxygen-binding affinity of the others
Juang RH (2004) BCbasics](https://image.slidesharecdn.com/haemoglobinmyoglobinstructure-220424180659/85/HAEMOGLOBIN-MYOGLOBIN-STRUCTURE-pptx-8-320.jpg)





![0 20 40 60 80 100 120
100
80
60
40
20
0
Percent
O
2
saturation
Partial pressure of oxygen (pO2, mmHg)
Muscle in
exercising
Relaxing
muscle Myoglobin
Hemoglobin
Artery
[O2]
Vein
[O2]
Environmental Oxygen Effects Binding Affinity
Adapted
from
Garrett
&
Grisham
(1999)
Biochemistry
(2e)
p.480](https://image.slidesharecdn.com/haemoglobinmyoglobinstructure-220424180659/85/HAEMOGLOBIN-MYOGLOBIN-STRUCTURE-pptx-17-320.jpg)