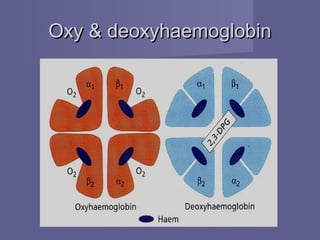
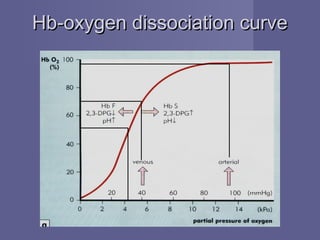
Haemoglobin is a protein in red blood cells that transports oxygen from the lungs to tissues and carbon dioxide from tissues back to the lungs. It is composed of haem and globin subunits. Globin is produced in polyribosomes while haem is produced in mitochondria and the two subunits combine to form functional haemoglobin. Abnormalities in haemoglobin structure or levels can impair oxygen delivery and cause anemia.