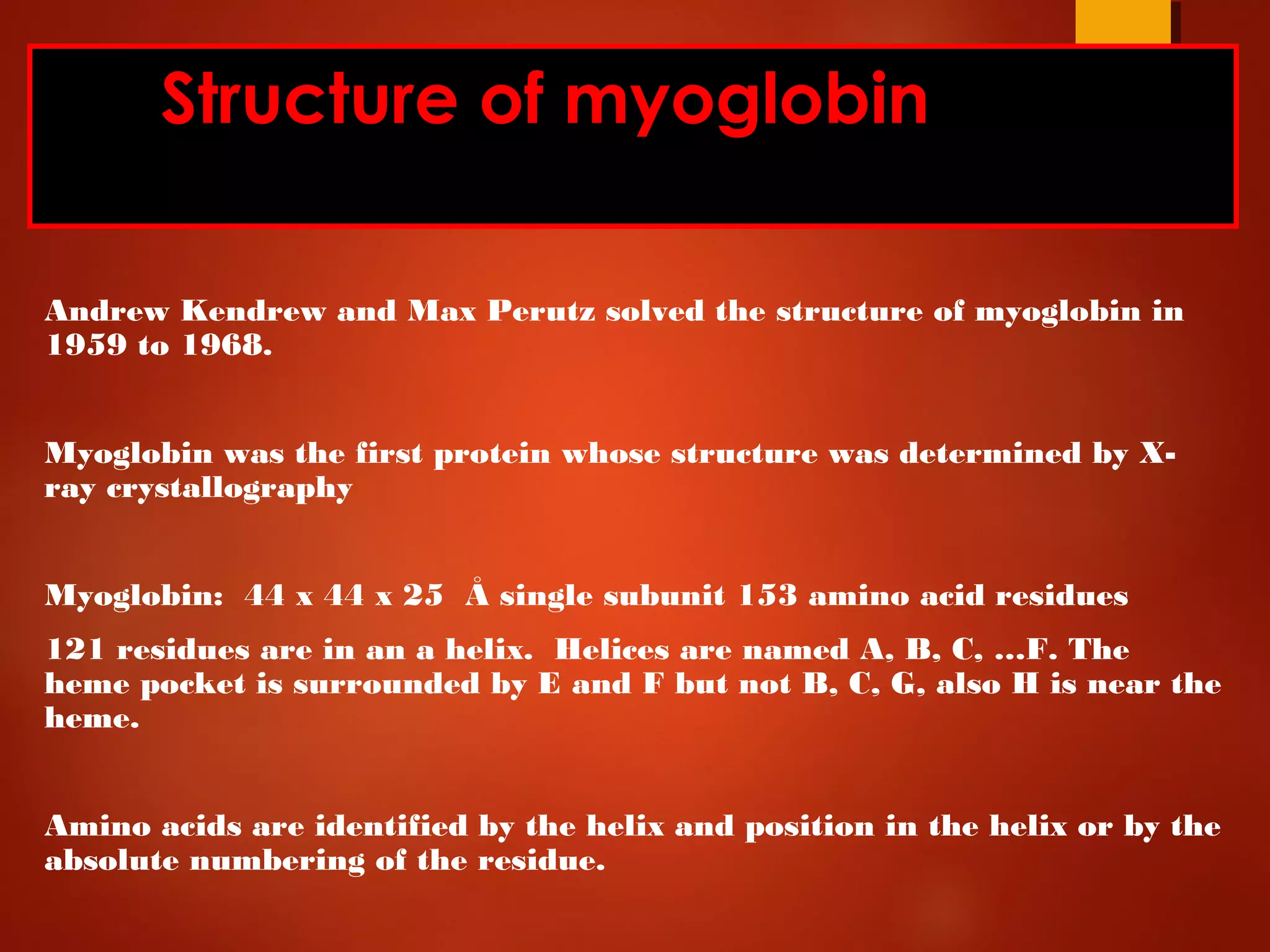
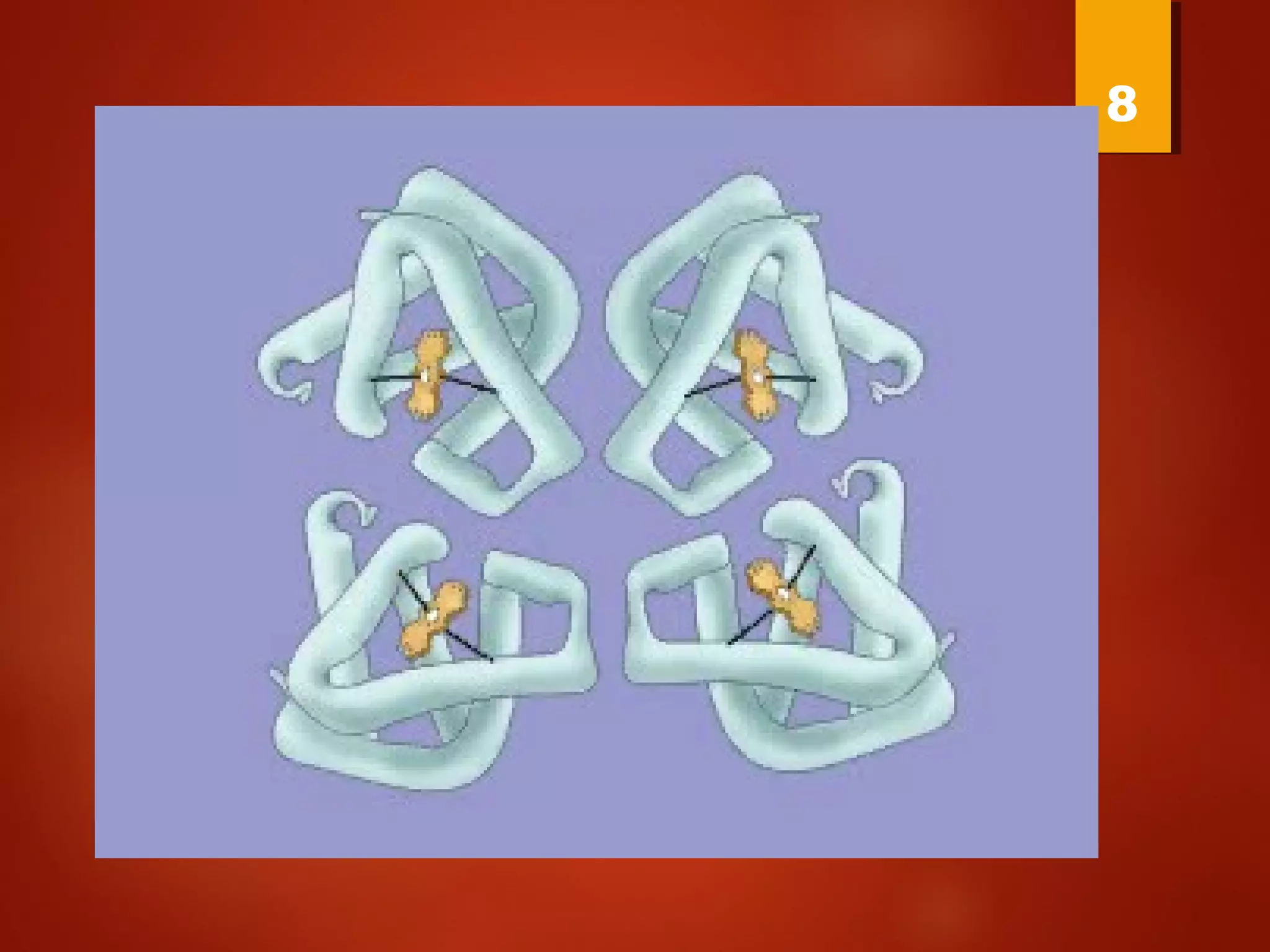
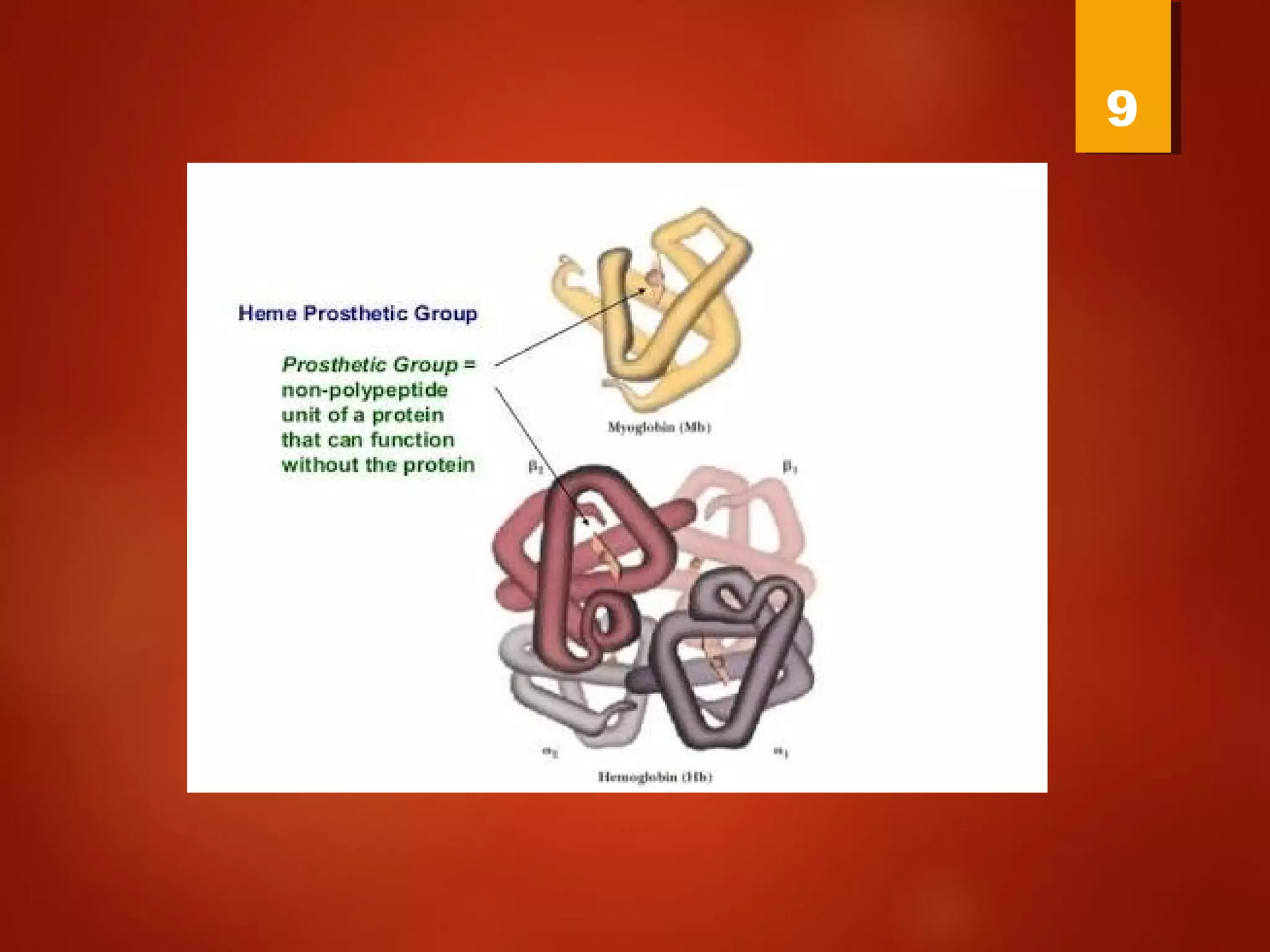
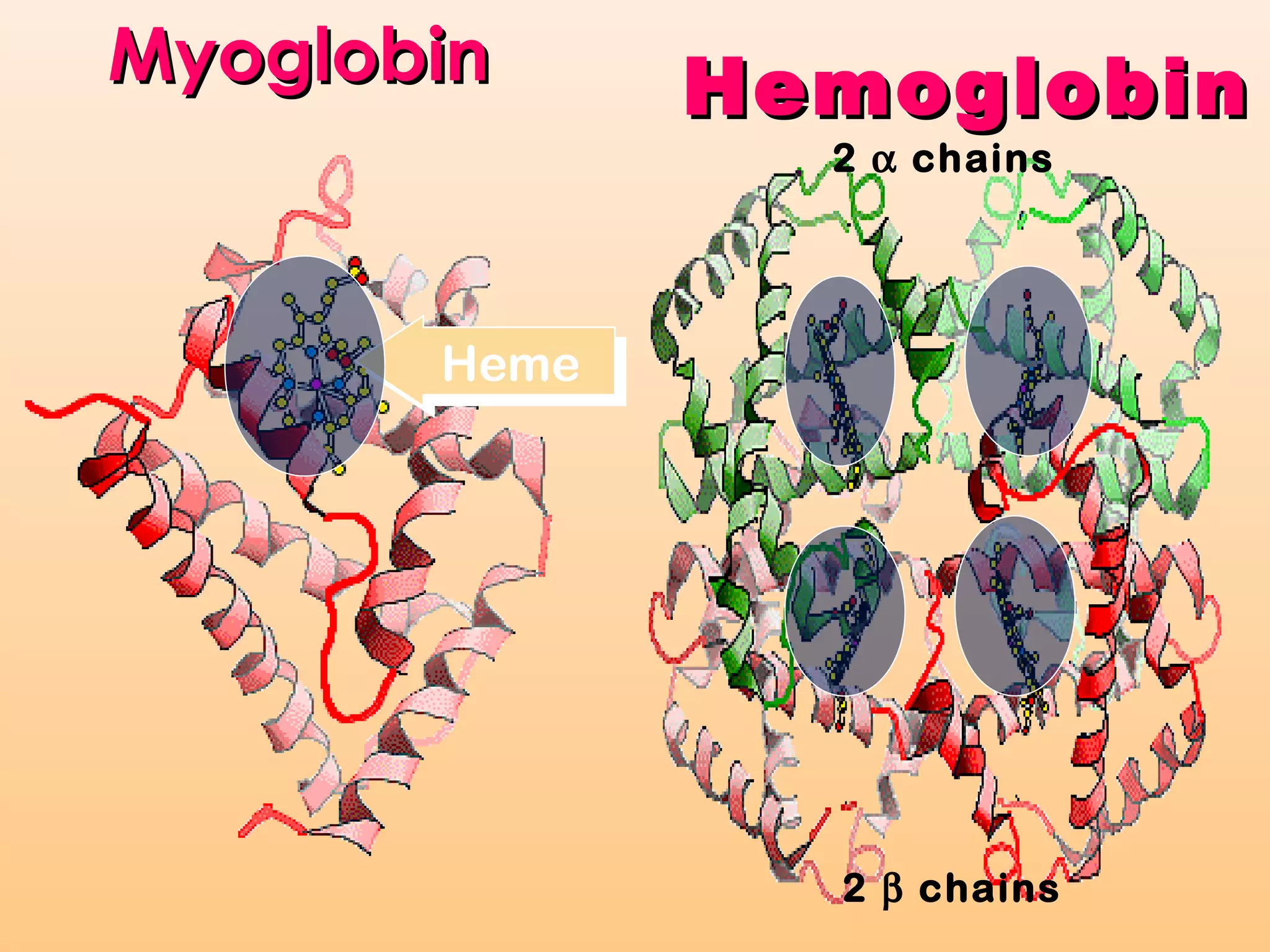
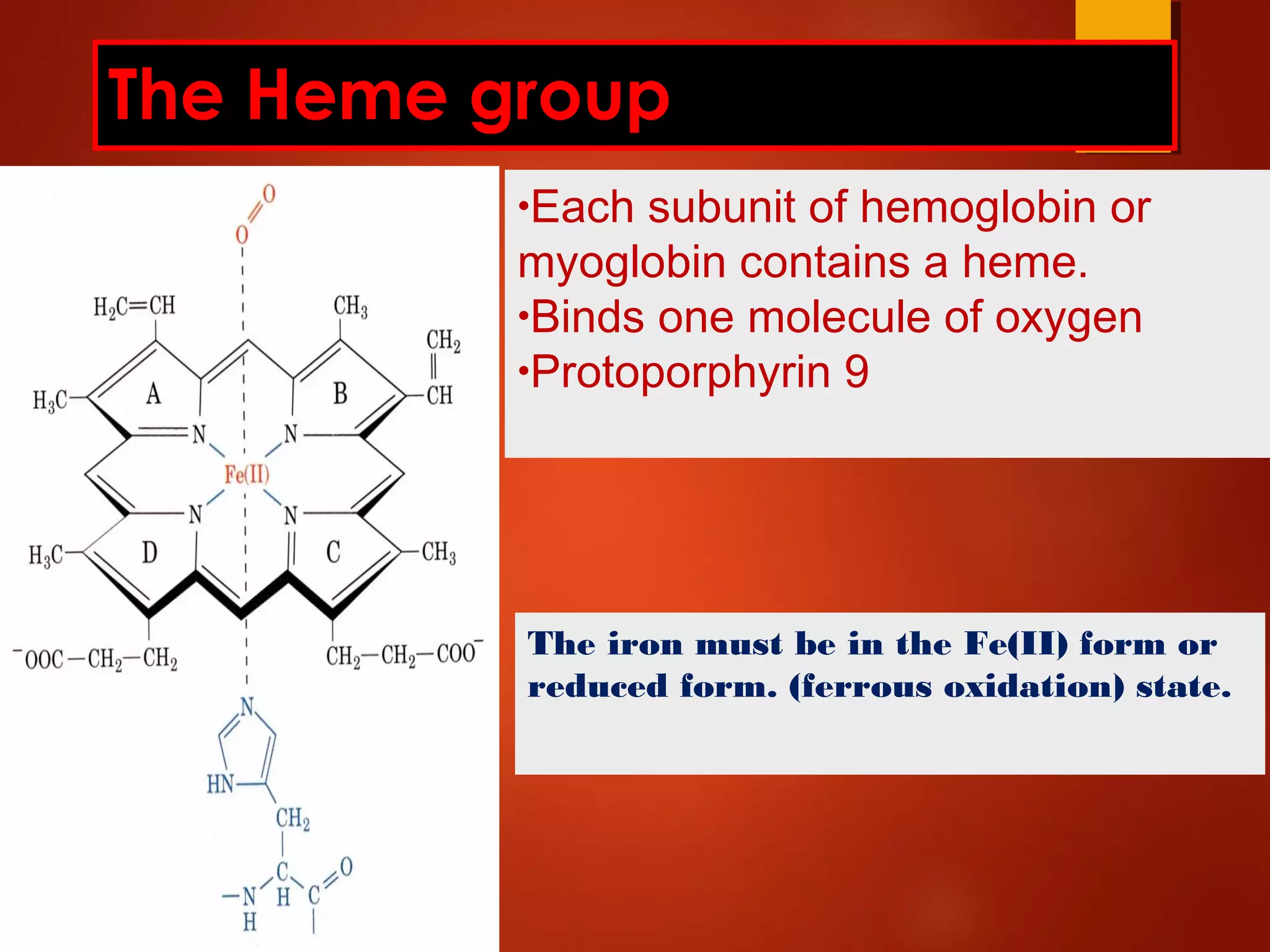
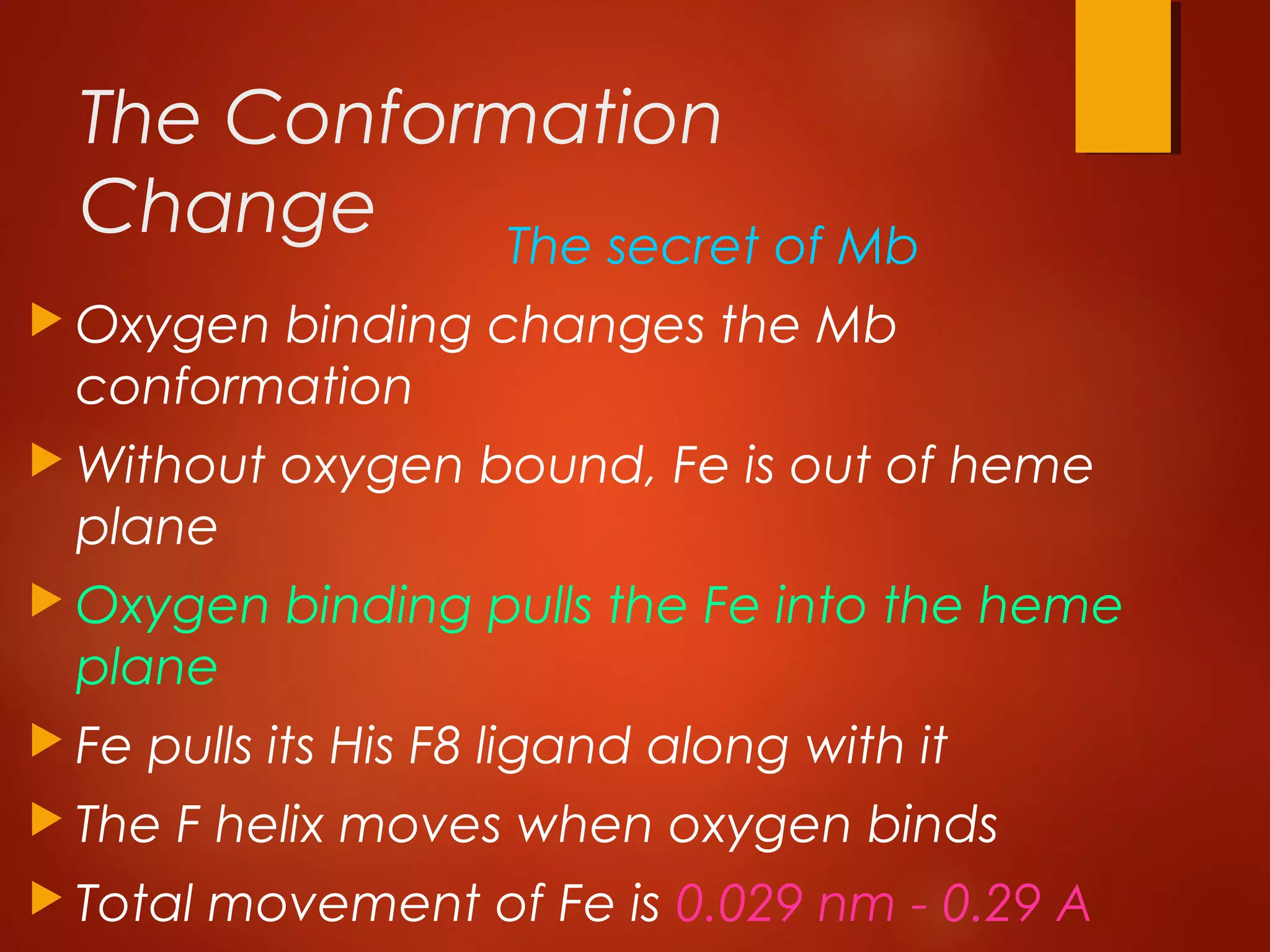
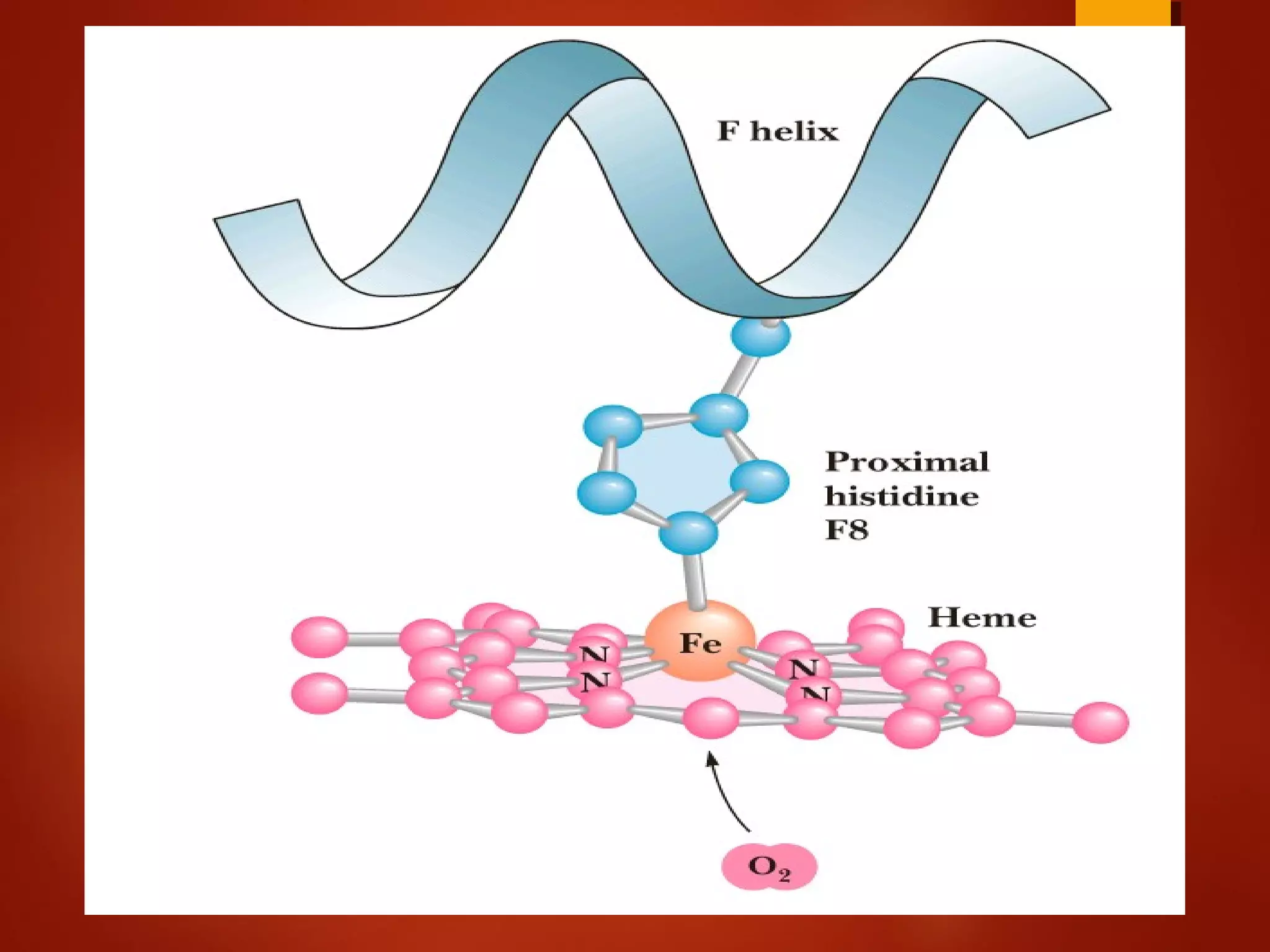
Myoglobin is a protein found in muscle tissue that binds oxygen. It was the first protein whose three-dimensional structure was determined using X-ray crystallography in the 1950s-60s. Myoglobin facilitates oxygen transport within muscles through reversible binding of oxygen to an iron-containing heme group. It stores oxygen to help meet rapid energy demands in muscle cells and prevents accumulation of toxic nitric oxide.
















![ Myoglobin an iron containing protein in muscles
,receive oxygen from rbc and transports it to the
mitochondria of muscle cells,where oxygen is used in
cellular respiration to produce energy
Oxymyoglobin –oxygen supply and act as scavenger of
NO[MYOCYTES]
Oxymyoglobin +NO= Harmless nitrates with ferric
myoglobin ,which is recycled by metmyoglobin
reductase
25](https://image.slidesharecdn.com/hemoglobinrwt-141012000056-conversion-gate01-170121070842/75/myoglobin-25-2048.jpg)


![O2 binding to myoglobin
22 MbOOMb ↔+
][MbO
][Mb][O
Kd
2
2
=
][OKd
][O
][MbO[Mb]
][MbO
Y
2
2
2
2
O2
+
=
+
=
Written backwards
we can get the
dissociation
constant
Fractional Saturation solve for [MbO2]
and plug in](https://image.slidesharecdn.com/hemoglobinrwt-141012000056-conversion-gate01-170121070842/75/myoglobin-28-2048.jpg)




![Diseases
Acute renal failure
Heart attack
Myoglobinuria [rhabdomyolysis]
33](https://image.slidesharecdn.com/hemoglobinrwt-141012000056-conversion-gate01-170121070842/75/myoglobin-33-2048.jpg)




