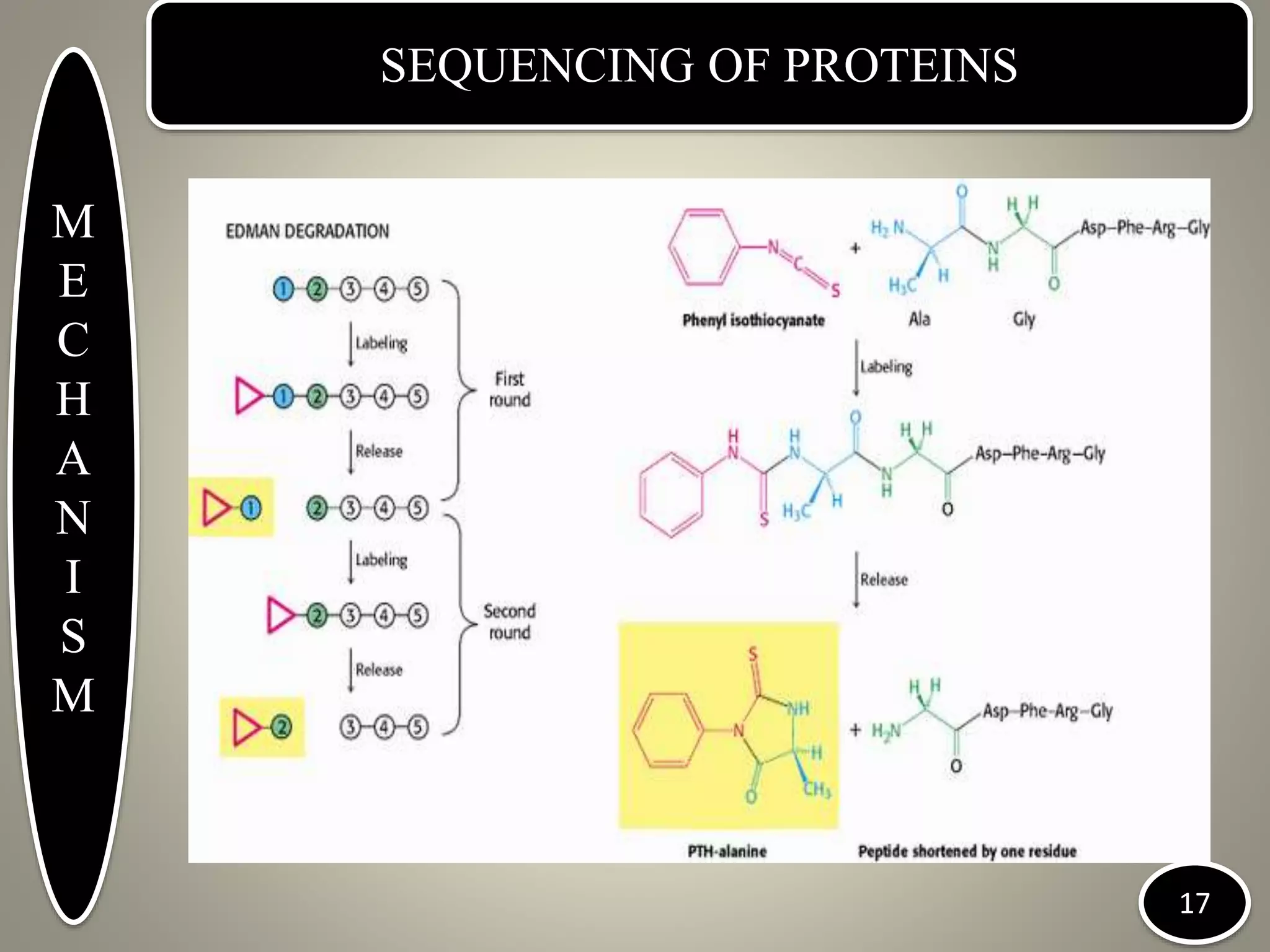
The document discusses protein sequencing techniques. It begins with an introduction to proteins and protein sequencing. The history of protein sequencing is then outlined, including early work by Edman and Sanger. Methods for determining amino acid composition through hydrolysis, separation, and analysis are described. The mechanisms of Sanger's method using dinitrophenyl reagents and Edman degradation using phenylisothiocyanate are explained. Applications of protein sequencing include determining protein structure and function. The document concludes with locations of protein sequencing institutes in India and abroad.