Embed presentation
Downloaded 76 times







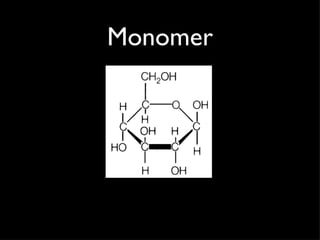
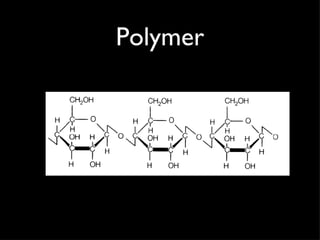

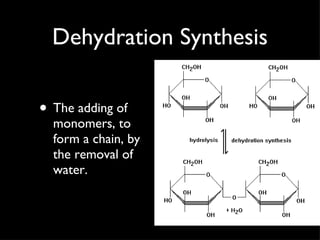
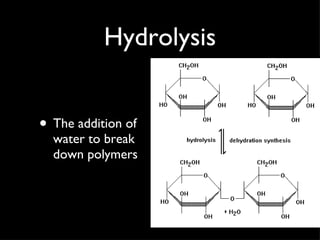

Organic compounds contain carbon and hydrogen, and may contain other elements like oxygen, nitrogen, and phosphorus. They make up most of living things. Inorganic compounds do not contain carbon or hydrogen. Carbon forms four bonds and can link to itself to form chains or rings, allowing it to combine with other elements to form the basic structures of organic molecules. Functional groups attached to carbon skeletons give molecules different properties and include amino, carboxyl, and phosphate groups. The four main organic compounds for life are carbohydrates, proteins, lipids, and nucleic acids. Macromolecules found in living things are large polymer molecules formed from combining smaller subunits through polymerization.












