organic and inorganic chemistry
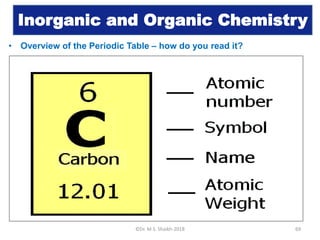
- 1. Inorganic and Organic Chemistry • Overview of the Periodic Table – how do you read it? ©Dr. M.S. Shaikh-2018 69
- 2. Inorganic and Organic Chemistry • Overview of the Periodic Table – what is atomic number? ©Dr. M.S. Shaikh-2018 70 • The number of protons found in the nucleus of an atom. Or • The number of electrons surrounding the nucleus of an atom.
- 3. Inorganic and Organic Chemistry • Overview of the Periodic Table – what is symbol? ©Dr. M.S. Shaikh-2018 71 An abbreviation of element name
- 4. Inorganic and Organic Chemistry • Overview of the Periodic Table – what is atomic weight? ©Dr. M.S. Shaikh-2018 72 The number of protons and neutrons in the nucleus of an atom
- 5. Inorganic and Organic Chemistry • Overview of the Periodic Table – how to find number of protons, electrons and neutrons in an element using periodic table? ©Dr. M.S. Shaikh-2018 73 • Number of protons = Atomic number • Number of electrons = Atomic number • Number of neutrons = Atomic weight – atomic number
- 6. Inorganic and Organic Chemistry • Overview of the Periodic Table – what is an element? ©Dr. M.S. Shaikh-2018 74 • A substance composed of a single kind of atom. • Cannot be broken down into another substance by chemical or physical means.
- 7. Inorganic and Organic Chemistry • Overview of the Periodic Table – what is a compound & mixture? ©Dr. M.S. Shaikh-2018 75 • A substance in which two or more different elements are chemically bonded together. • Two or more substances that are mixed together but are not chemically bonded.
- 8. Inorganic and Organic Chemistry • Overview of the Periodic Table – Example of element, compound and mixture ©Dr. M.S. Shaikh-2018 76 • Sodium is an element. • Chlorine is an element. • When sodium and chlorine bond they make the compound sodium chloride, commonly known as table salt. • When water is added, it becomes mixture. • Compounds have different properties than the elements that make them up. • Table salt has different properties than sodium, an explosive metal, and chlorine, a poisonous gas.
- 9. Inorganic and Organic Chemistry • Periodic Table – more details ©Dr. M.S. Shaikh-2018 77 • The periodic table organizes the elements in a particular way. • A great deal of information about an element can be gathered from its position in the periodic table. • For example, you can predict with reasonably good accuracy the physical and chemical properties of the element. • You can also predict what other elements a particular element will react chemically. • Understanding the organization and plan of the periodic table will help you obtain basic information about each of the 118 known elements.
- 10. Inorganic and Organic Chemistry • Periodic Table – more details – continue… ©Dr. M.S. Shaikh-2018 78 • Elements are organized on the table according to their atomic number, usually found near the top of the square. • The atomic number refers to how many protons an atom of that element has. • For instance, hydrogen has 1 proton, so it’s atomic number is 1. • The atomic number is unique to that element. • No two elements have the same atomic number.
- 11. Inorganic and Organic Chemistry • Periodic Table – what is a square in the table? ©Dr. M.S. Shaikh-2018 79 • Different periodic tables can include various bits of information, but usually: • Atomic number • Symbol • Atomic mass • Number of valence electrons • State of matter at room temperature.
- 12. Inorganic and Organic Chemistry • Periodic Table – valence electrons ©Dr. M.S. Shaikh-2018 80 • The number of valence electrons an atom has may also appear in a square. • Valence electrons are the electrons in the outer energy level of an atom. • These are the electrons that are transferred or shared when atoms bond together.
- 13. Inorganic and Organic Chemistry • Periodic Table – Categories ©Dr. M.S. Shaikh-2018 81 • The elements of periodic table are divided into three categories. • Metals • Non Metals • Metalloids
- 14. Inorganic and Organic Chemistry • Periodic Table – Categories - Metals ©Dr. M.S. Shaikh-2018 82 • Metals are good conductors of heat and electricity. • Metals are shiny. • Metals are ductile (can be stretched into thin wires). • Metals are bendy (can be pounded into thin sheets). • A chemical property of metal is its reaction with water which results in corrosion.
- 15. Inorganic and Organic Chemistry • Periodic Table – Categories - Non Metals ©Dr. M.S. Shaikh-2018 83 • Non-metals are poor conductors of heat and electricity. • Non-metals are not ductile or malleable. • Solid non-metals are brittle and break easily. • They are dull/ weak. • Examples . . .
- 16. Inorganic and Organic Chemistry • Periodic Table – Categories - Metalloids ©Dr. M.S. Shaikh-2018 84 • Metalloids (metal-like) have properties of both metals and non-metals. • They are solids that can be shiny or dull. • They conduct heat and electricity better than non-metals but not as well as metals. • They are ductile and malleable. • Examples…
- 17. Inorganic and Organic Chemistry • Periodic Table – Arrangements ©Dr. M.S. Shaikh-2018 85 • Elements in periodic table are grouped into: • Families / groups – vertical columns • Elements in families have similar properties. • Periods / series – horizontal rows • Elements in periods do not have similar properties.
- 18. Inorganic and Organic Chemistry • Periodic Table – Arrangements – continue… ©Dr. M.S. Shaikh-2018 86 • Columns of elements are called groups or families. • Elements in each family have similar but not identical properties. • For example, lithium (Li), sodium (Na), potassium (K), and other members of family IA are all soft, white, shiny metals. • All elements in a family have the same number of valence electrons. • Each horizontal row of elements is called a period. • The elements in a period are not alike in properties. • In fact, the properties change greatly across given row. • The first element in a period is always an extremely active solid. • The last element in a period, is always an inactive gas. Families Periods
- 19. Inorganic and Organic Chemistry Periodic Table A lot of more information to be covered by: • Self reading... • Students presentations… ©Dr. M.S. Shaikh-2018 87
- 20. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 88 • Few concepts… Solution? • Mixture of two or more substances in a single phase. • Once constituent is regarded as solvent and the other as solute. • Solutions are homogeneous mixtures of two or more pure substances. • In a solution, the solute is dispersed uniformly throughout the solvent.
- 21. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 89 • Few concepts… Solute? • The part of a solution that is being dissolved. • Usually the lesser in amount.
- 22. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 90 • Few concepts… Solvent? • The part of a solution that dissolves the solute. • Usually the greater in amount.
- 23. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 91 • Few concepts Solute + Solvent = Solution? Solute Solvent
- 24. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 92 • Few concepts… Miscible substances? • Liquids that will dissolve in each other.
- 25. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 93 • Few concepts… Electrolytes? • Liquids that are capable of conducting electricity.
- 26. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 94 • Types of solutions • Liquid solutions - salt and water, sugar and water • Gaseous solutions - Air and other mixture of gases • Solid solutions - combination of solids, homogenous mixture of metals (alloys)
- 27. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 95 • Types of solutions – concentration terms • Concentrated solutions – solutions with lots of solute per solvent • Dilute solutions – solutions with lots of solvent. • Saturated solutions – solutions with maximum amount of solute • Super saturated solutions – solution with more solute than it can hold.
- 28. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 96 • Types of solutions – concentration terms Dilute solution Concentrated solution
- 29. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 97 • Types of solutions – concentration terms
- 30. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 98 • Types of solutions – concentration terms Super saturated solution • A solution that contains more solute than it can theoretically hold at a given temperature. • If more solute is added in the solution, the formation of crystals increases. • This solution type is unstable.
- 31. Inorganic and Organic Chemistry To be continued… ©Dr. M.S. Shaikh-2018 99
- 32. Inorganic and Organic Chemistry • Chemistry of solutions – how solutions are formed? ©Dr. M.S. Shaikh-2018 100 • Solvent molecules attracted to surface ions. • Each ion is surrounded by solvent molecules. • Enthalpy changes with each interaction (broken / formed).
- 33. Inorganic and Organic Chemistry • Chemistry of solutions – how solutions are formed? ©Dr. M.S. Shaikh-2018 101 • The ions are solvated (surrounded by solvent). • If the solvent is water, the ions are hydrated. • The intermolecular forces.
- 34. Inorganic and Organic Chemistry • Chemistry of solutions – how solutions are formed? ©Dr. M.S. Shaikh-2018 102 • Solvation • It is an interaction of a solute with the solvent, which leads to stabilization of the solute species in the solution. • In the solvated state, an ion in a solution is surrounded or complexed by solvent molecules.
- 35. Inorganic and Organic Chemistry • Chemistry of solutions – how solutions are formed? ©Dr. M.S. Shaikh-2018 103 • Solvation – an example of salt and water
- 36. Inorganic and Organic Chemistry • Chemistry of solutions – how solutions are formed? ©Dr. M.S. Shaikh-2018 104 • Solvation – rate of solvation – the influencing parameters • Stirring / shaking • Breaking apart a solute • Heating the solvent / temperature
- 37. Inorganic and Organic Chemistry • Chemistry of solutions – how solutions are formed? ©Dr. M.S. Shaikh-2018 105 How does a solid dissolve in a liquid? This gives a concept of dissolution. What is dissolution?
- 38. Inorganic and Organic Chemistry • Chemistry of solutions – Dissolution? ©Dr. M.S. Shaikh-2018 106 • A process by which a solid, liquid or gas forms a solution in a solvent. • In solids, this can be explained as the breakdown of crystal lattice into individual ions, atoms or molecules and their transport into the solvent.
- 39. Inorganic and Organic Chemistry • Chemistry of solutions – Dissolution? ©Dr. M.S. Shaikh-2018 107 • For liquids and gases, the molecules must be compatible with those of solvent for a solution to form. • In this process, solid substance solubilizes in a given solvent.
- 40. Inorganic and Organic Chemistry • Chemistry of solutions – Dissolution – example … ©Dr. M.S. Shaikh-2018 108
- 41. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 109 Dissolution versus reaction • Dissolution is a physical change. • Original solute can get back by evaporating the solvent in dissolution process. • However, it is not possible to get back original solute in a reaction.
- 42. Inorganic and Organic Chemistry • Chemistry of solutions ©Dr. M.S. Shaikh-2018 110 Solubility • Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. • It is measured in terms of the maximum amount of solute (expressed in grams) dissolved in a solvent (100 grams) at equilibrium (specific temperature and pressure). • The resulting solution is called a saturated solution.
- 43. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 111 Factors affecting solubility • Temperature • Pressure • Molecular size • Stirring • Intermolecular attraction
- 44. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 112 • Effect of temperature on solubility • Generally, the solubility of solid solute in liquid solvents increases with increase in temperature. • It is obvious from graph that the solubility increases with respect temperature.
- 45. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 113 • Effect of temperature on solubility There is no simple relationship between the structure of a substance and the temperature dependence of its solubility.
- 46. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 114 • Effect of temperature on solubility – other examples…
- 47. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 115 • Effect of temperature on solubility of gases • The solubility of gases in liquids decreases with increasing temperature. • This is a reverse of the case of solids. • Attractive intermolecular interactions in the gas phase are essentially zero for most substances.
- 48. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 116 • Effect of temperature on solubility of gases
- 49. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 117 • Effect of temperature on solubility of gases • When a gas dissolves, it does so because its molecules interact with solvent molecules. • Because heat is released when these new attractive interactions form. • Dissolving most gases in liquids is an exothermic process. • Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas.
- 50. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 118 • Effect of temperature on solubility of gases • The carbonated soft drinks have more bubbles when they are in refrigerator. • As temperature increases, gas molecules gain more kinetic energy and are able to leave a solution.
- 51. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 119 • Effect of temperature on solubility of gases Cold
- 52. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 120 • Effect of pressure solubility • The solubility of liquids and solids does not change appreciably with pressure. • But a solubility of gas in a liquid is directly proportional • to its pressure. • The concentration of gas molecules increases with increasing pressure. • The concentration of dissolved gas molecules in the solution at equilibrium is also higher at higher pressures.
- 53. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 121 • Effect of pressure solubility • The figure shows why the solubility increases with respect to pressure of gas.
- 54. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 122 • Effect of pressure solubility • (a) When a gas comes in contact with a pure liquid, some of the gas molecules (purple spheres) collide with the surface of the liquid and dissolve. • When the concentration of dissolved gas molecules has increased so that the rate at which gas molecules escape into the gas phase is the same as the rate at which they dissolve. • A dynamic equilibrium has been established, as depicted in figure.
- 55. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 123 • Effect of pressure solubility • This equilibrium is entirely similar to the one that maintains the vapor pressure of a liquid. • (b) Increasing the pressure of the gas increases the number of molecules of gas per unit volume, which increases the rate at which gas molecules collide with the surface of the liquid and dissolve. • (c) As additional gas molecules dissolve at the higher pressure, the concentration of dissolved gas increases until a new dynamic equilibrium is established.
- 56. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 124 • Effect of pressure solubility • The relationship between pressure and the solubility of a gas is described quantitatively by Henry’s law, which is named for its discoverer, the English (British) physician and chemist, William Henry (1803).
- 57. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 125 • Effect of pressure solubility - Henry’s law • Henry's law is one of the gas laws and was formulated by the British physician / chemist, William Henry, in 1803. “The solubility of a gas in a liquid is directly proportional to the pressure of the gas”
- 58. Henry’s Law ©Dr. M.S. Shaikh-2018 126 • Henry's law has two major assumptions. • Two major assumptions: 1. For the system is at low pressure. 2. For the species present as a very dilute solutions in liquid phase – low concentration. Inorganic and Organic Chemistry
- 59. Henry’s Law – Other statements ©Dr. M.S. Shaikh-2018 127 • The partial pressure of the species in the vapour phase is directly proportional to its liquid-phase mole fraction. • At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. ( )NiHxPy iii ,...,2,1== pressureTotal: constantsHenry': fractionmolephase: fractionmolephase: P H Vy Lx i i i − − Inorganic and Organic Chemistry
- 60. Henry’s Law – continue ©Dr. M.S. Shaikh-2018 128 • Moreover, according to the Henry's law, the pressure of the gas is also proportional to the solubility of gas in a particular solution / liquid mixture at any constant temperature. • The concentration of a solute gas in a solution is directly proportional to the partial pressure of the gas over the solution. This can also be represented as: P = KHC • Henry’s law is used to calculate the concentration of a gas in the solution under pressure. P is the partial pressure of the gas above the solution KH is the Henry’s law constant for the solution C is the concentration of the dissolved gas in solution Inorganic and Organic Chemistry
- 61. Henry’s Law constant ©Dr. M.S. Shaikh-2018 129 • Henry's law constant is an expression for equilibrium distribution of a compound between liquid (water) and gaseous phase. • Denoted by KH. • Expressed as: KH = P / C KH = atm / (mol / lit) C = mol / lit P = atm • Different for every gas, temperature and solvent. Inorganic and Organic Chemistry
- 62. Henry’s Law – Validity and Limitations ©Dr. M.S. Shaikh-2018 130 • Henry's law is only an approximation that is applicable for dilute solutions. • Henry’s law is obeyed fairly satisfactorily by many gases of low solubility, provided the pressure is not too high or the temperature is not too low. • The dissolved gas always follows the Henry’s law. • The law is strictly applicable to those gases where the molecular species are the same in the liquid phase. Inorganic and Organic Chemistry
- 63. Henry’s Law – Validity and Limitations ©Dr. M.S. Shaikh-2018 131 • It also applies simply for solutions where the solvent does not react chemically with the gas being dissolved. • Suggests that, Henry's law is related with physical absorption only. • The gas phase should not undergo any chemical change. • Large deviations from Henry’s law are observed in case of gases of high solubility and particularly those which interact with the liquid solvent. Inorganic and Organic Chemistry
- 64. Henry’s Law – Applications ©Dr. M.S. Shaikh-2018 132 • Soft drink industries • Natural gas processing industries • Various gas – liquid processes in chemical industries • Miscellaneous Inorganic and Organic Chemistry
- 65. Henry’s Law – example problem ©Dr. M.S. Shaikh-2018 133 How many grams / quantity of carbon dioxide gas is dissolved in a 1 litre bottle of carbonated water if the manufacturer uses a pressure of 2.4 atm in the bottling process at 25 °C? Given data: KH of CO2 in water = 29.76 atm/(mol/L) at 25 °C P = 2.4 atm; We know the equation: P = KHC Therefore : C = P / KH Put these values… 2.4 / 29.76 = > 0.0806 mol / lit of CO2 Convert it into grams… Inorganic and Organic Chemistry
- 66. Henry’s Law – example problem ©Dr. M.S. Shaikh-2018 134 • 0.0806 mol / lit of CO2 (convert it into grams) • 1 mole of CO2 = 12 + (16 × 2) = 12 + 32 = 44 g • Mass of CO2 in grams = moles of CO2 × 44 g / mol • Therefore : = 0.0806 mol × 44 g / mol = 3.546 g There are 3.546 g of CO2 dissolved in a 1 litre bottle of carbonated water from the manufacturer. Molecular weight of CO2 Inorganic and Organic Chemistry
- 67. Henry’s Law – exercise problems…for you ©Dr. M.S. Shaikh-2018 135 Inorganic and Organic Chemistry To understand why soft drinks “fizz” and then go “flat” after being opened, calculate the concentration of dissolved CO2 in a soft drink. The soft drink is bottled under a pressure of 5.0 atm of CO2. In equilibrium with the normal partial pressure of CO2 in the atmosphere. The Henry’s law constant for CO2 in water at 25°C is 3.4×10−2 M/atm.
- 68. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 136 • Effect of molecular size on solubility • The larger the molecules of solute, the larger will be their molecular weight and their size. • It is more difficult for solvent molecules to surround the bigger molecules. • Therefore, the larger molecules / particles are generally less soluble.
- 69. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 137 • Effect of stirring on solubility • Stirring only increase the speed of the process. • It increases the movement of the solvent that exposes the solute, thus enabling the solubility. • As molecules in the liquid substances are in constant move, the process would take place any way, but it would take more time.
- 70. Inorganic and Organic Chemistry • Chemistry of solutions – solubility ©Dr. M.S. Shaikh-2018 138 • Effect of intermolecular attraction on solubility • The stronger the intermolecular attraction between solute and solvent, the more likely the solute will dissolve. • Example: ethanol in water, glucose in water – more soluble. • Cyclohexane: not a water soluble.
- 71. ©Dr. M.S. Shaikh-2018 139 • The study of various solutions / fluids / mixtures used in the chemical process industries is very essential. • The solution properties especially, physical / physicochemical / thermophysical / thermodynamic properties of the solutions / mixtures are very necessary to study. • These properties can be of pure substances and the mixtures of various substances. • These properties are very important for the design, operation and optimization of chemical processes. Inorganic and Organic Chemistry • Chemistry of solutions – the solution properties
- 72. ©Dr. M.S. Shaikh-2018 140 • There are various properties of the solutions as below: • Density • Viscosity • Surface tension • Refractive index • Conductivity • Resistivity • Vapour pressure Fundamental design properties Inorganic and Organic Chemistry • Chemistry of solutions – the solution properties…
- 73. ©Dr. M.S. Shaikh-2018 141 • Most of the materials in a real world are not pure substances, but a mixture of various types of substances. • The pure substances from which solutions are prepared, are called as components / constituents of the solutions. • It is very important to study the properties of the pure components and mixture of various substances. • Since the temperature, pressure and compositions affects the solution properties, therefore, it is essential to study the effect of these parameters. Inorganic and Organic Chemistry • Chemistry of solutions – the solution properties…
- 74. ©Dr. M.S. Shaikh-2018 142 • Partial properties The property of component when it is in mixture of one or more other components. • Derived properties The property of the pure or mixture of solutions derived from another basic or fundamental properties. • Excess properties The difference between actual property value of the solution and the property value of the pure component. Inorganic and Organic Chemistry • Chemistry of solutions – the solution properties…