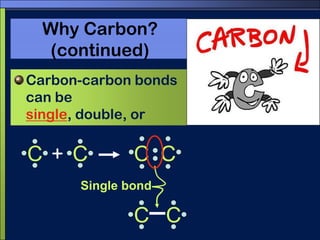
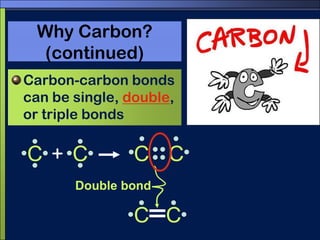
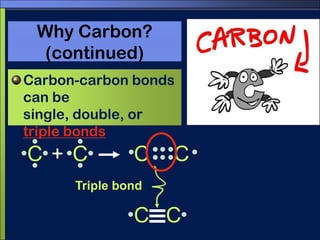
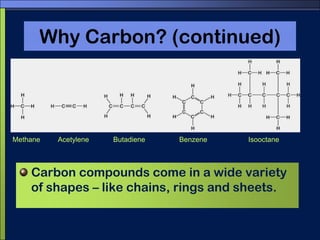
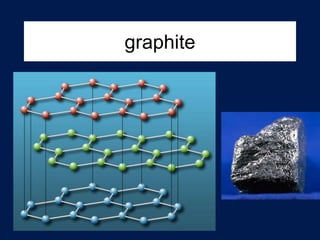
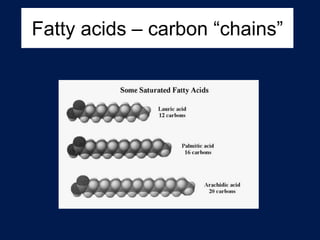
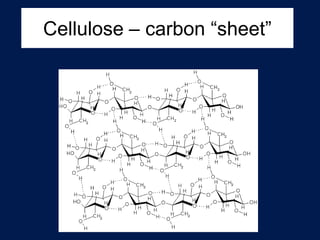
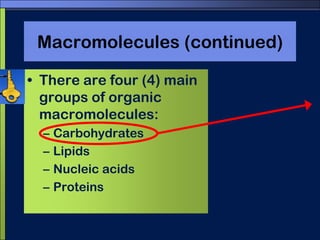
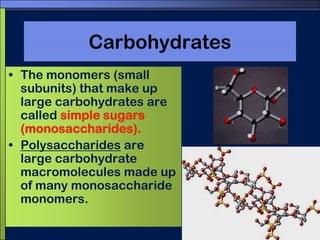
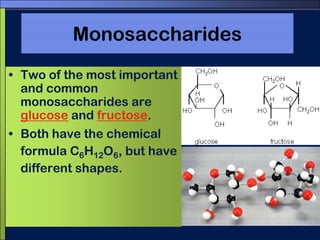
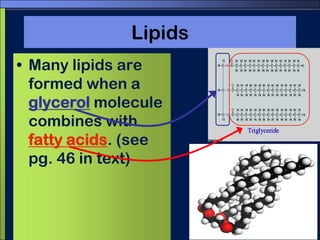
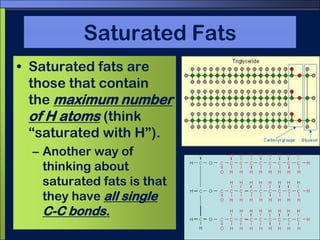
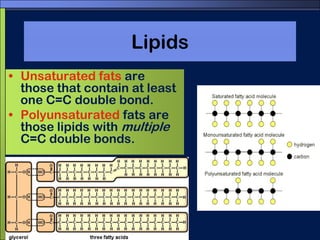
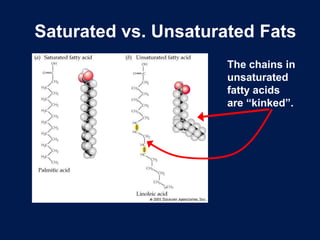
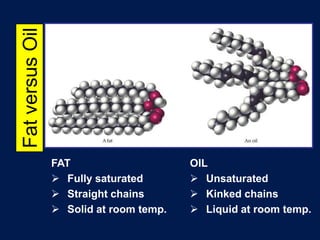
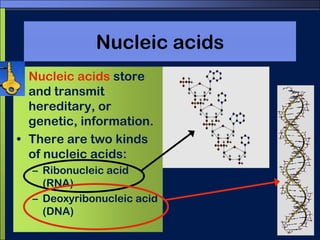
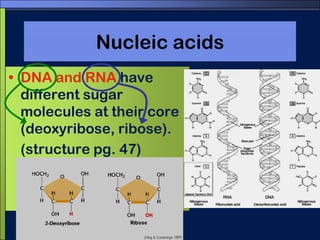
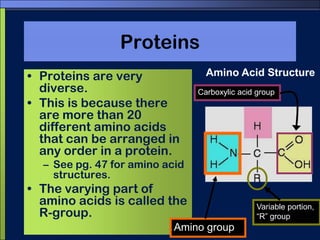
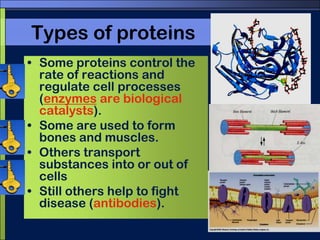
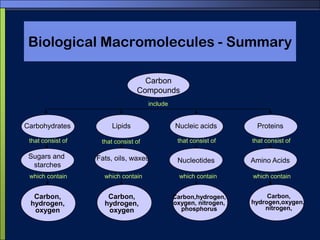
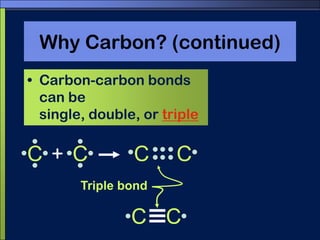
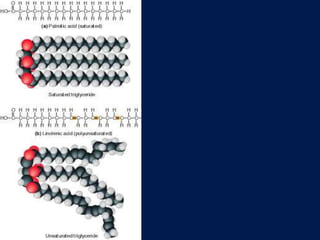
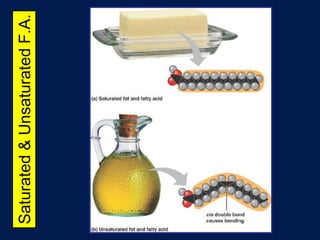
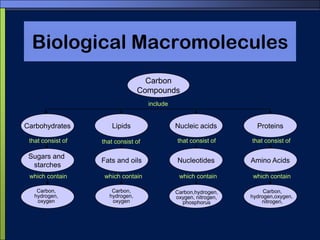
This document discusses carbon compounds and macromolecules that are essential for life. It explains that carbon can form strong bonds to other carbon atoms and a variety of elements, allowing it to make chains and complex molecules. The four main types of macromolecules are carbohydrates, lipids, nucleic acids, and proteins. Carbohydrates include sugars and starches, lipids include fats and oils, nucleic acids are made of nucleotides, and proteins consist of amino acids. These macromolecules contain carbon along with other elements and are critical components of living things.