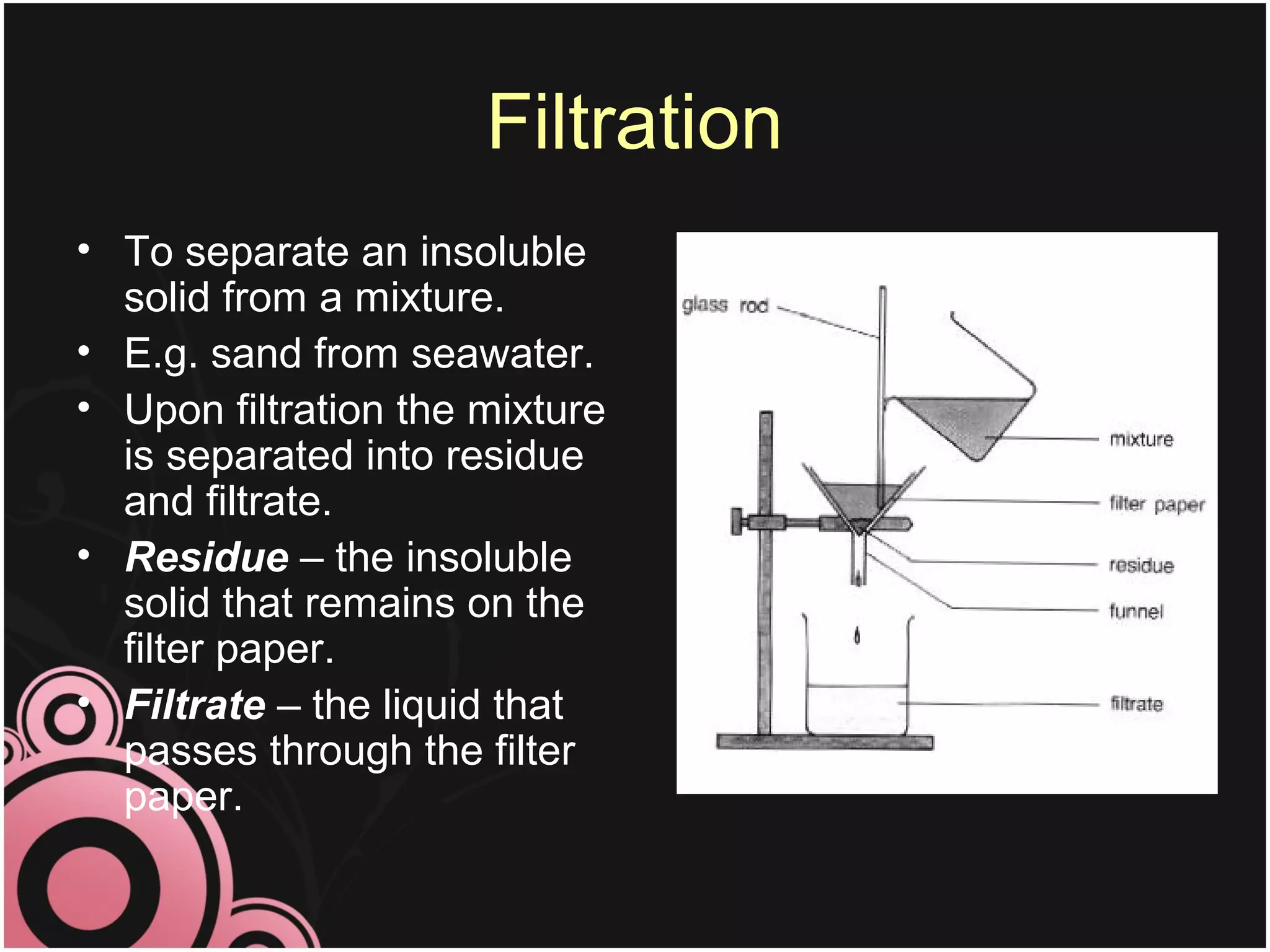
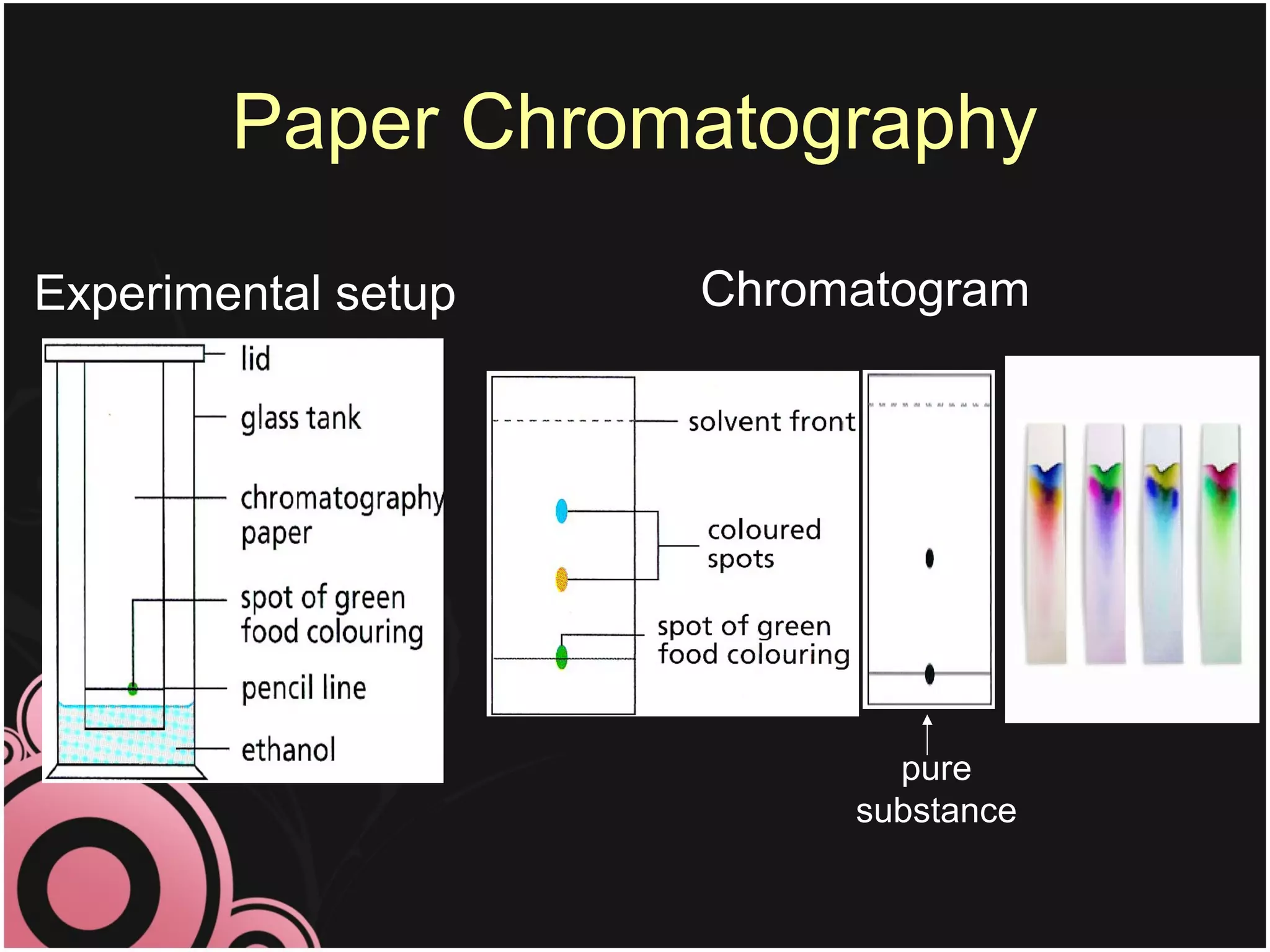
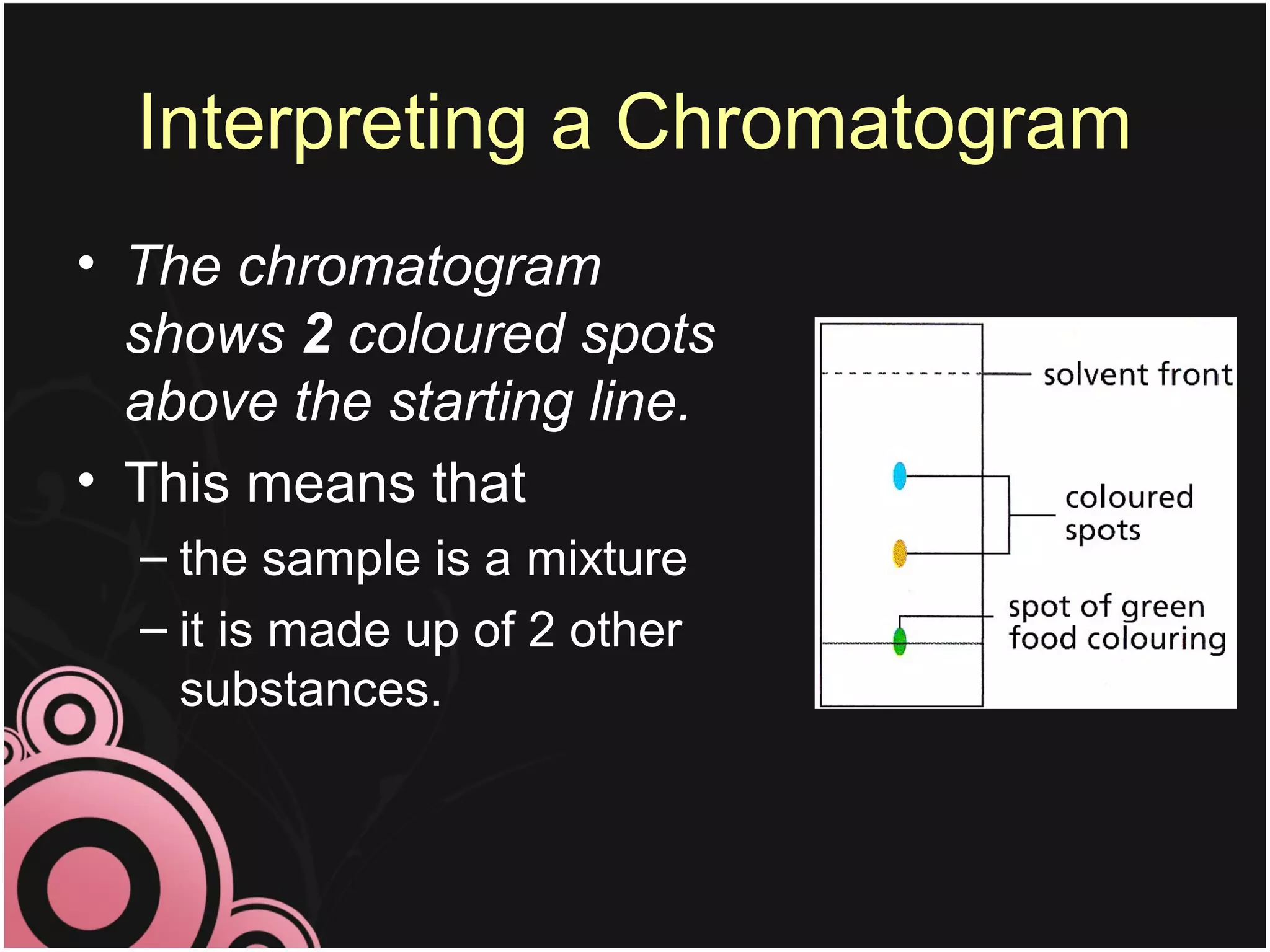
This document discusses methods for separating mixtures into their pure components. It describes techniques like filtration, crystallization, distillation, and paper chromatography. Filtration can separate insoluble solids from liquids. Crystallization involves evaporating a solution to leave behind crystals of the solute. Distillation separates liquids based on their boiling points. Paper chromatography uses a solvent to separate mixtures on a paper strip based on how far different substances travel up the paper. The document emphasizes that pure substances have fixed melting and boiling points, while mixtures can be separated into pure components using these techniques.