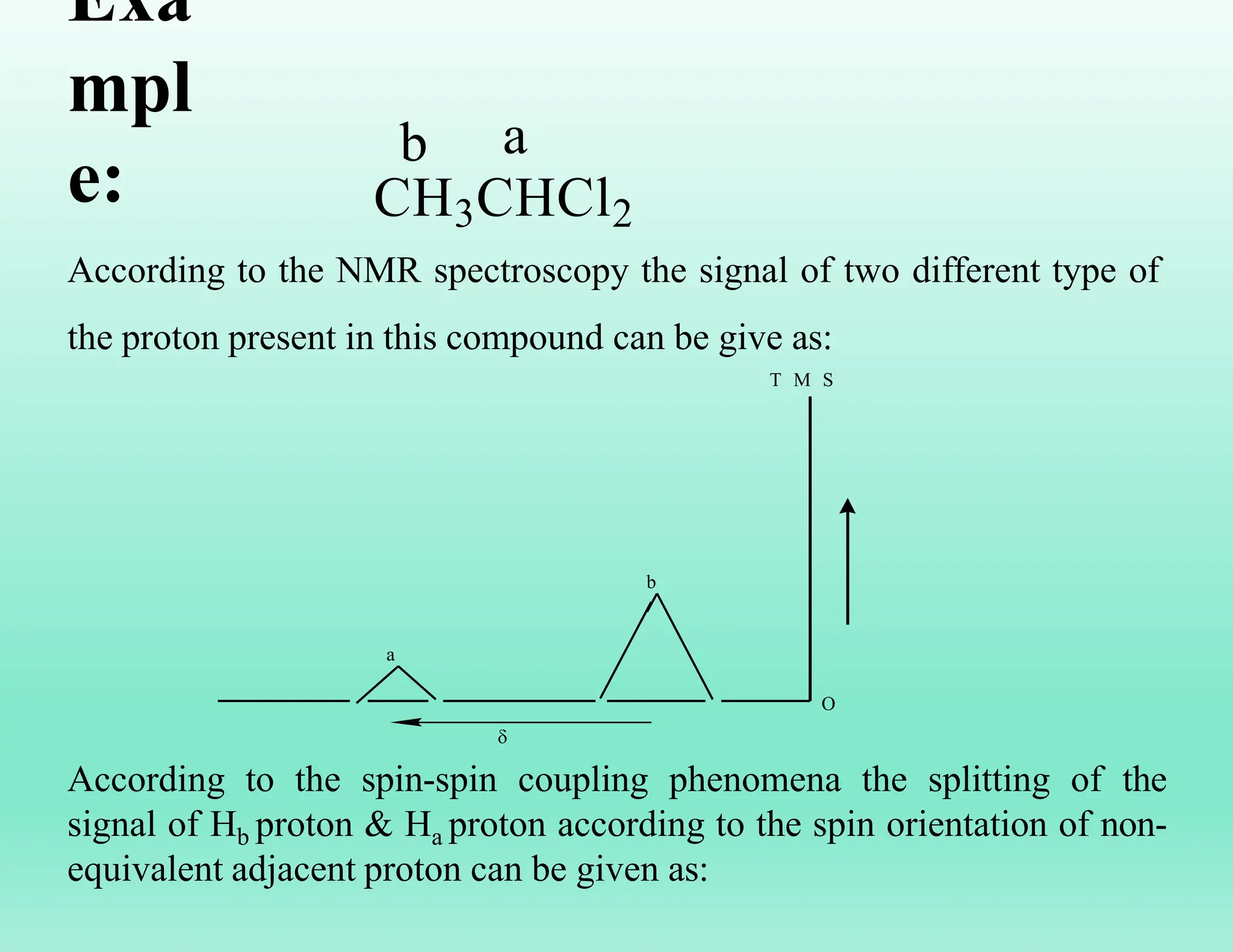
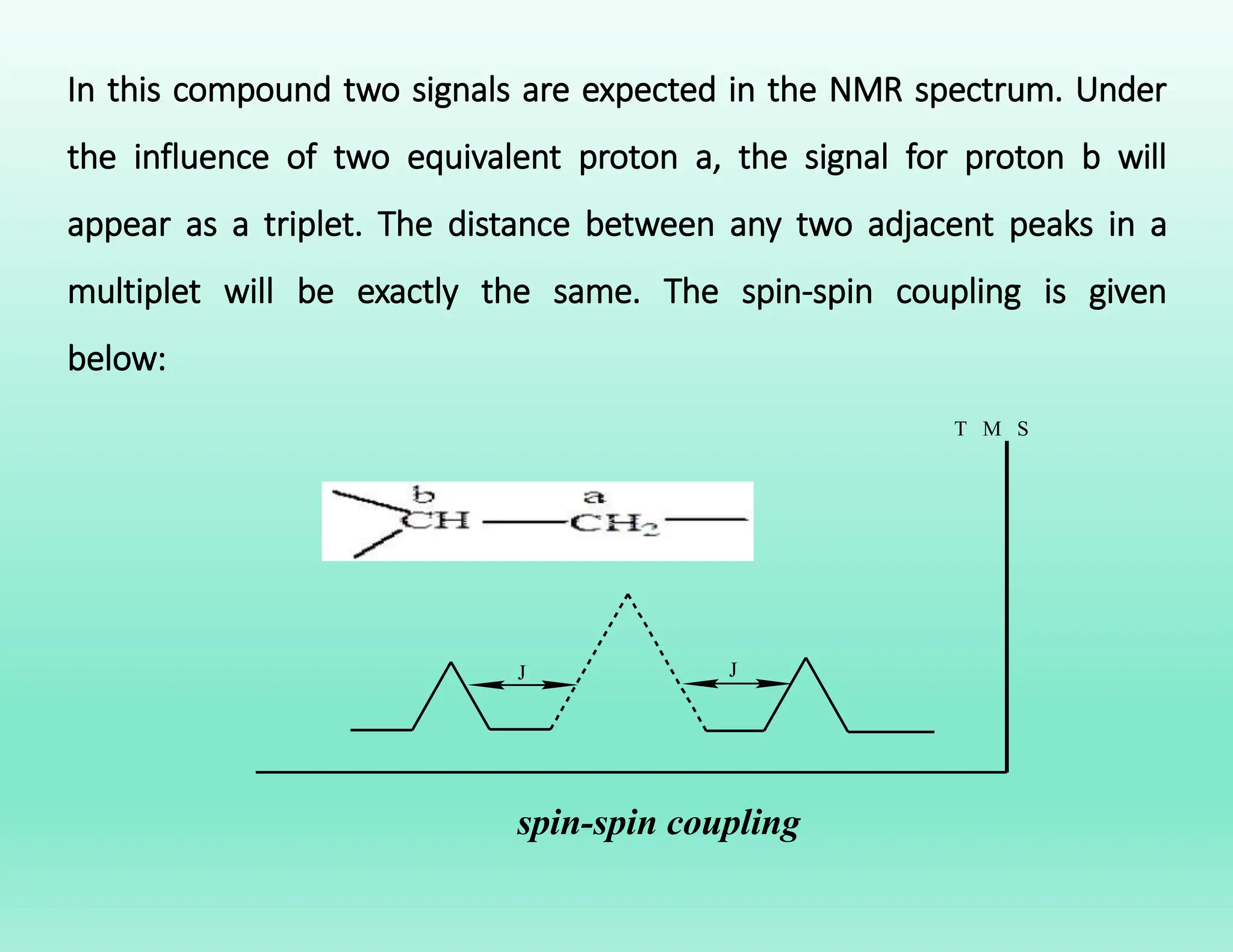
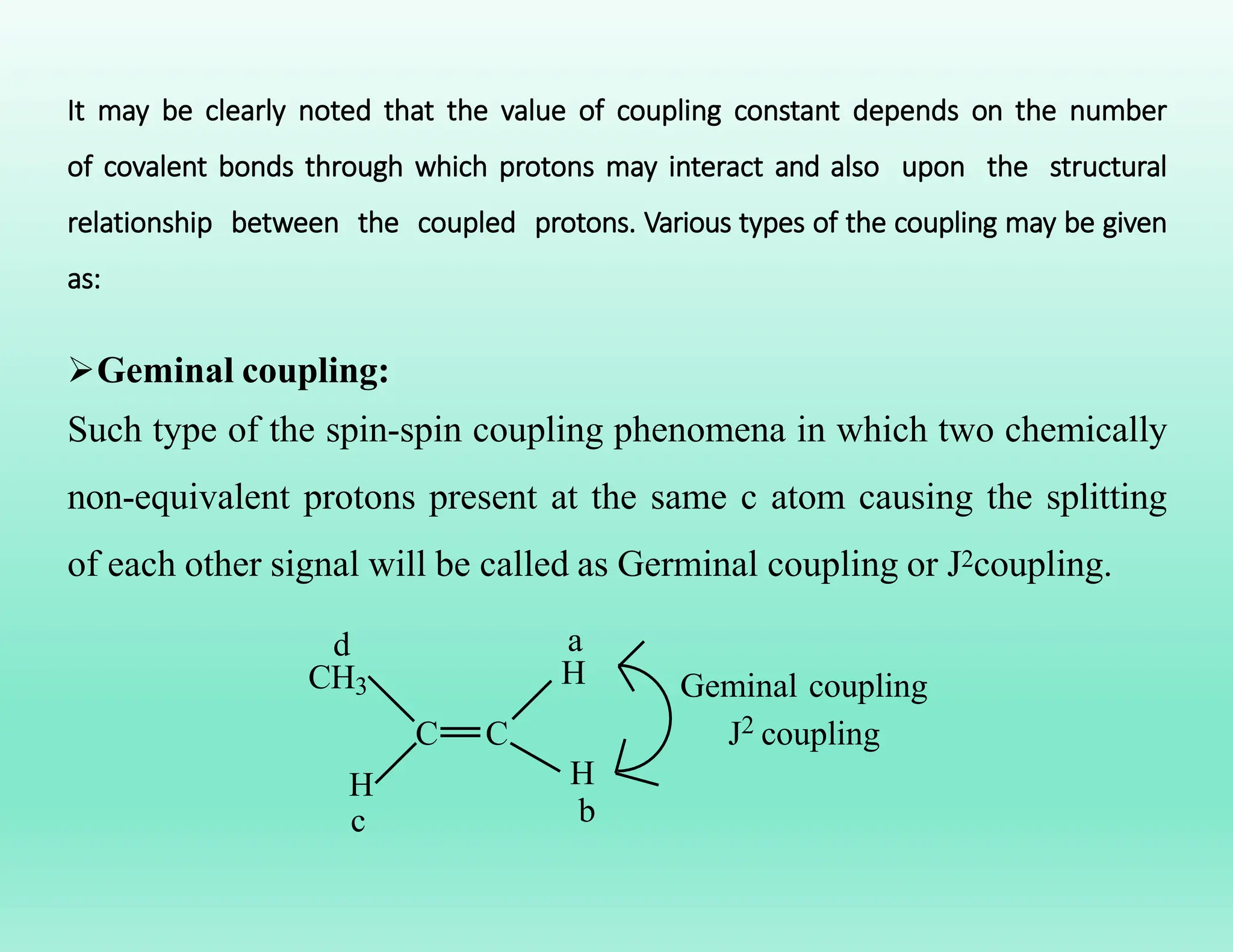
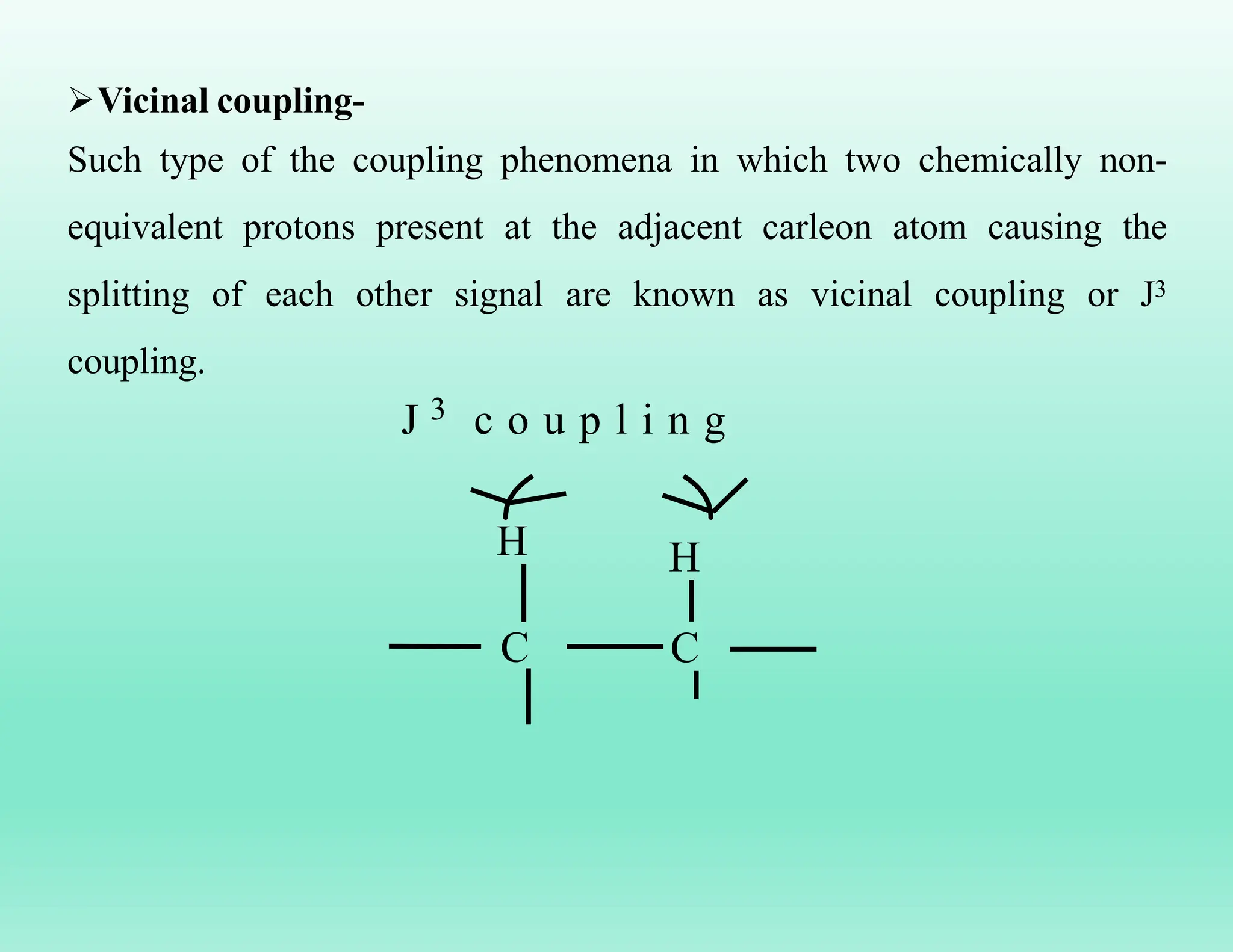
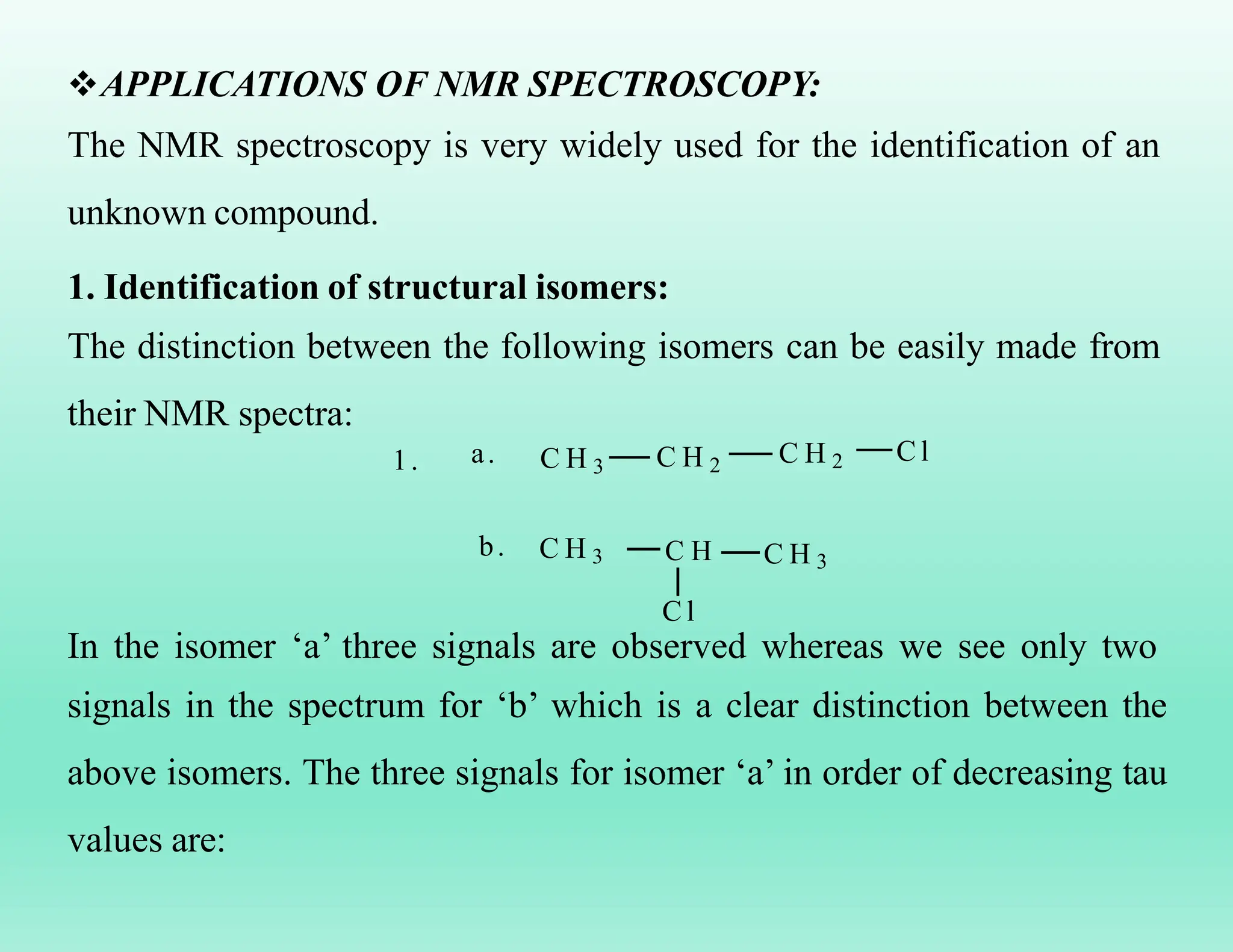
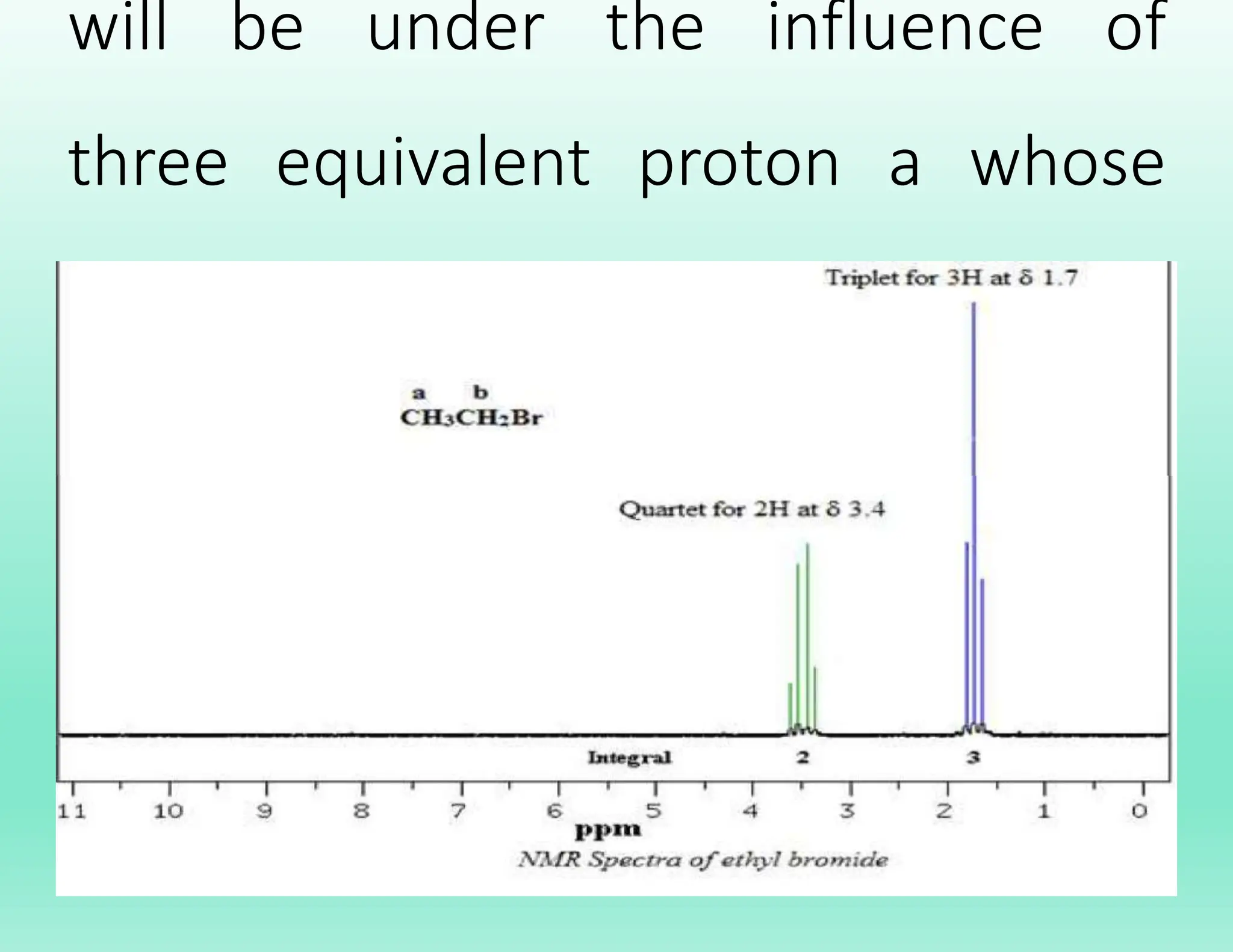
This document provides an overview of NMR spectroscopy. It begins by defining NMR spectroscopy and describing how nuclei absorb electromagnetic radiation when placed in a magnetic field. It then discusses key concepts like NMR active nuclei, chemical shielding and deshielding, chemically equivalent and non-equivalent protons, and spin-spin splitting and coupling constants. The document concludes by outlining some applications of NMR spectroscopy like identification of structural isomers and distinction between cis-trans isomers.