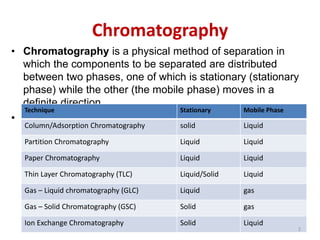
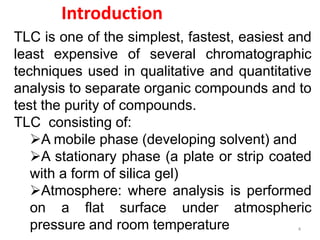
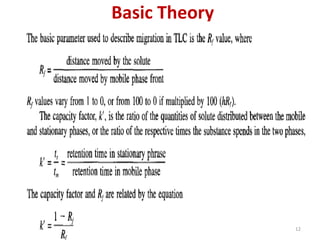
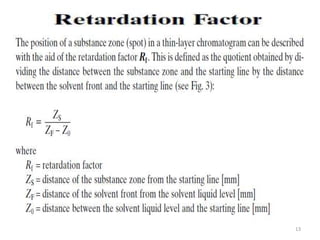
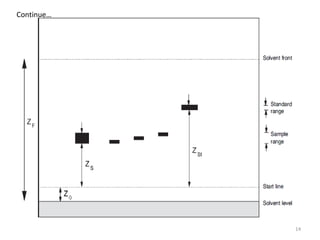
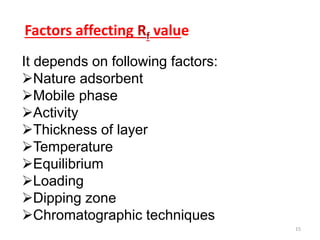
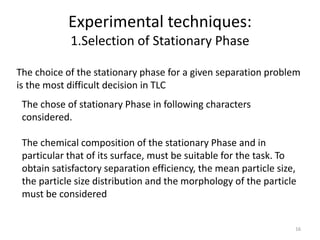
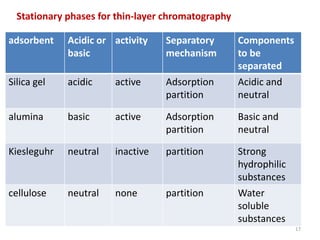
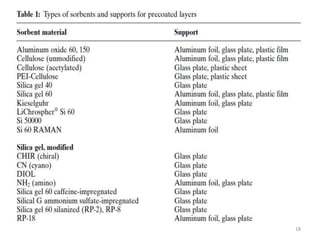
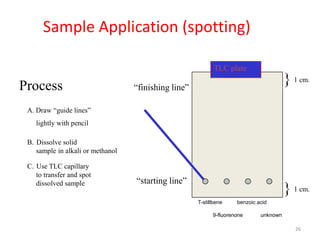
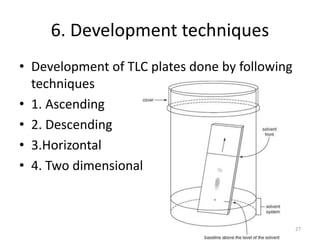
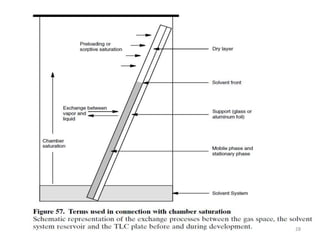
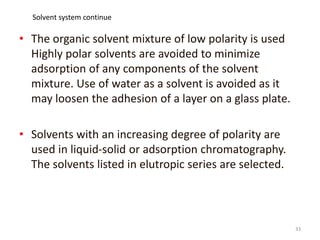
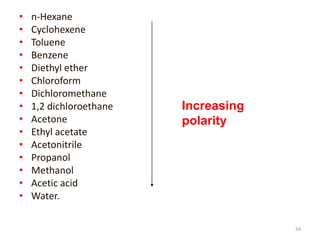
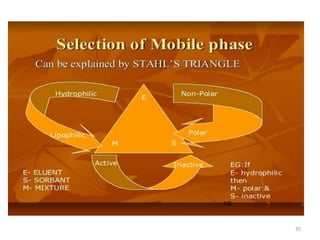
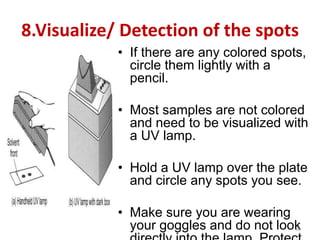
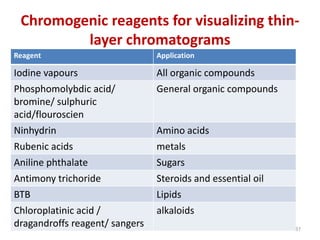
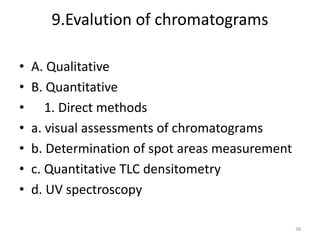
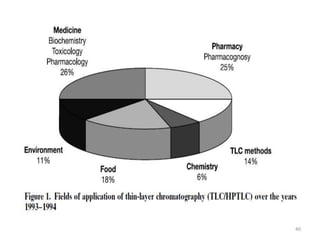
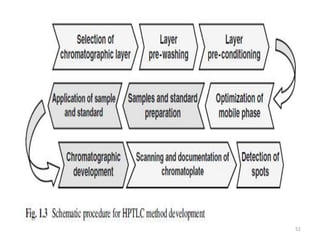
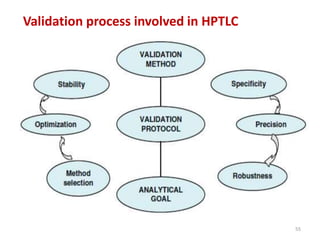
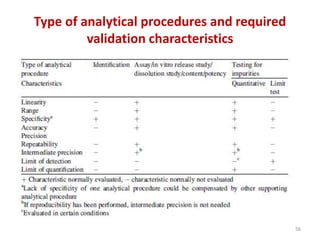
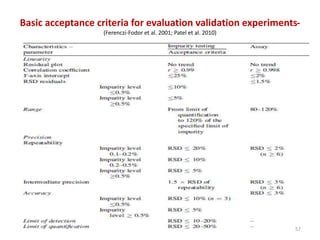
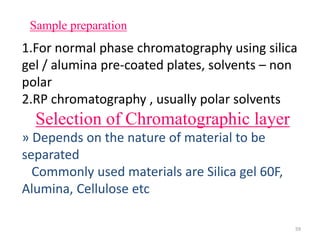
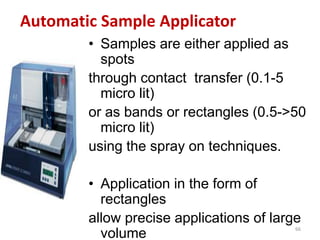
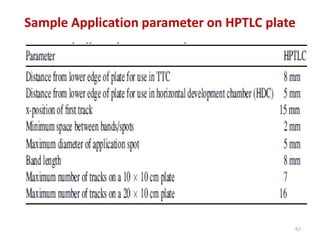
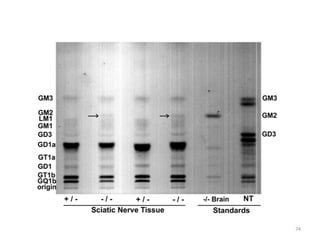
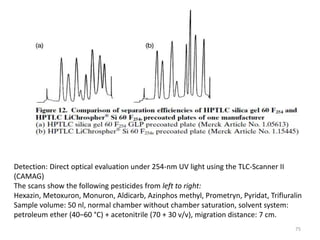
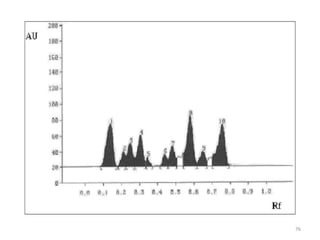
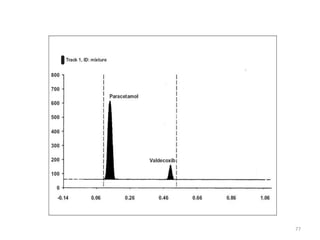
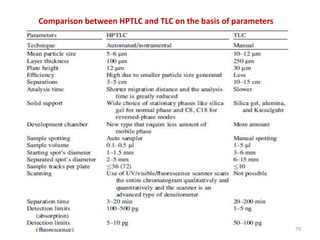
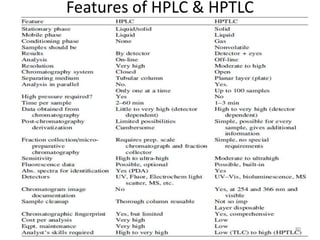
Thin layer chromatography (TLC) is a chromatography technique used to separate mixtures. It uses a stationary phase, typically silica gel on a glass or aluminum plate, and a mobile phase, usually a solvent or solvent mixture, to separate compounds in a mixture. High performance TLC (HPTLC) is an advanced form of TLC that provides better separation using optimized stationary phases with smaller particle sizes and tighter size distributions compared to normal TLC plates. The key steps in TLC and HPTLC involve preparing the chromatographic plate, applying the sample as a spot, developing the plate with a mobile phase, and detecting separated components using visualization techniques. TLC and HPTLC have various applications in analyzing organic compounds,