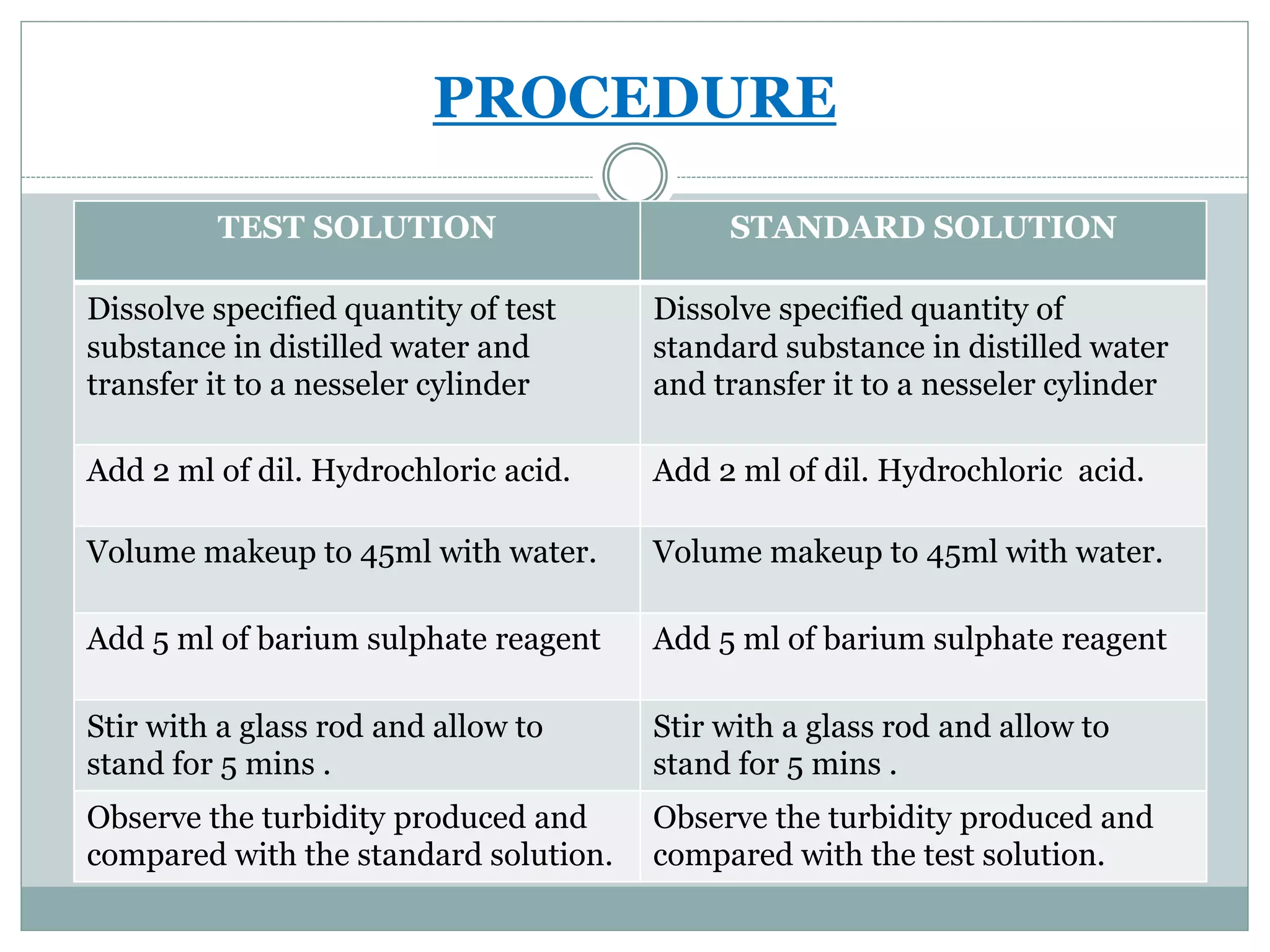
This document describes the limit test for sulfate. The test is based on the reaction between barium chloride and soluble sulfates in the presence of hydrochloric acid. This results in the precipitation of barium sulfate. The turbidity produced by the test solution is compared to that of a standard sulfate solution. If the turbidity of the test solution is less than the standard, then the sample passes the limit test for sulfate.