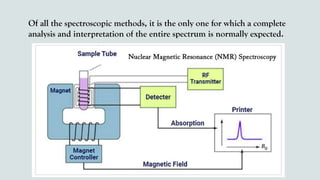
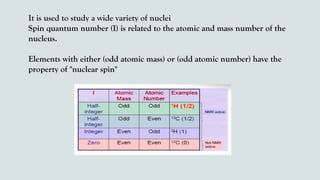
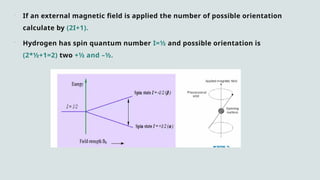
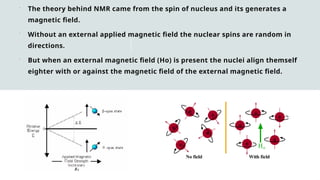
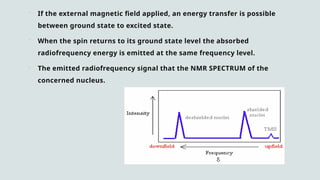
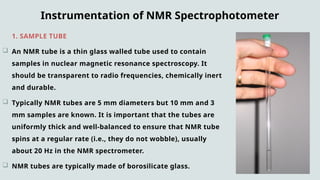
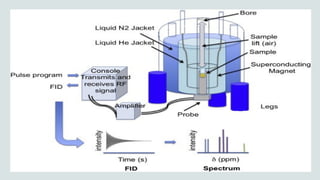
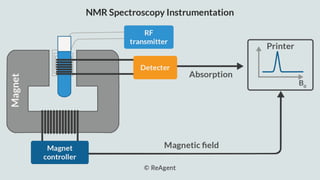
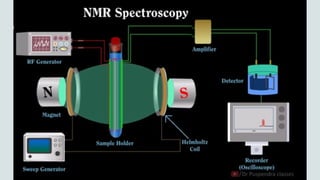
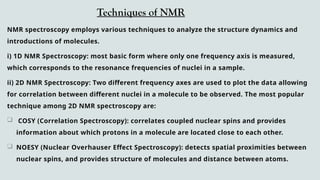
The document provides a comprehensive overview of Nuclear Magnetic Resonance (NMR) Spectroscopy, including its history, principles, instrumentation, applications, and analysis techniques. NMR is a crucial spectroscopic method used for determining the structure of organic compounds and is integral in various fields such as chemistry and biochemistry. Various NMR techniques like 1D, 2D, and solid-state spectroscopy are utilized for detailed molecular analysis.