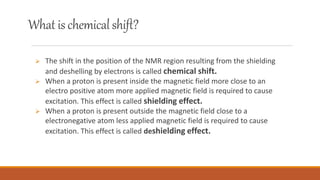
Nuclear magnetic resonance (NMR) spectroscopy is an analytical technique that uses the magnetic properties of certain atomic nuclei to determine the structure of organic molecules. NMR spectroscopy works by applying a strong magnetic field to a sample and using radio waves to excite the magnetic nuclei, then measuring the radio signals emitted as the nuclei relax. The two most common types of NMR are proton NMR and carbon-13 NMR. NMR spectroscopy has many applications in fields like chemistry, medicine, and biochemistry, allowing researchers to determine molecular structures, image tissues and organs, study metabolic processes, and more.