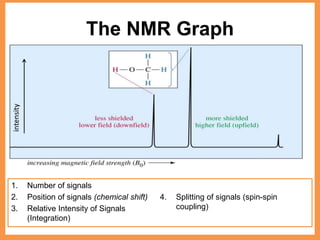
This document discusses NMR spectroscopy, which uses the magnetic properties of atomic nuclei. It explains that certain nuclei absorb and re-emit electromagnetic radiation at specific resonance frequencies depending on their magnetic properties and the strength of the magnetic field. NMR spectra provide information about the number of signals, chemical shifts, signal intensities, and splitting of signals due to spin-spin coupling between neighboring nuclei.