1. 1H NMR spectroscopy is a technique used to analyze compounds by detecting hydrogen nuclei in a magnetic field. It provides information about functional groups, number of nuclei, and structure of compounds.
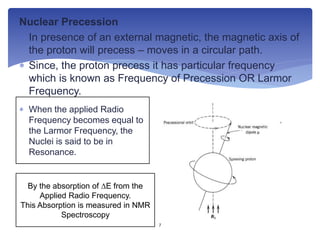
2. The principle involves hydrogen nuclei absorbing radio frequencies matching their Larmor frequency in an applied magnetic field. This absorption is measured to produce an NMR spectrum.
3. Factors like electronegativity, magnetic anisotropy, and spin-spin coupling influence the chemical shifts observed on the NMR spectrum, allowing identification of functional groups and structure elucidation.